Understanding the Cohort Data Set in AbbVie’s PIONEER Trials
The PIONEER I and II studies (ClinicalTrials.gov identifiers: NCT01468207 and NCT01468233) serve as pivotal investigations into the efficacy of adalimumab for managing hidradenitis suppurativa (HS). These trials provide critical data that fosters our understanding of treatment effects and biomarker associations within the patient population. Here’s a deep dive into the structure and insights from the cohort data set.
Patient Demographics and Study Design
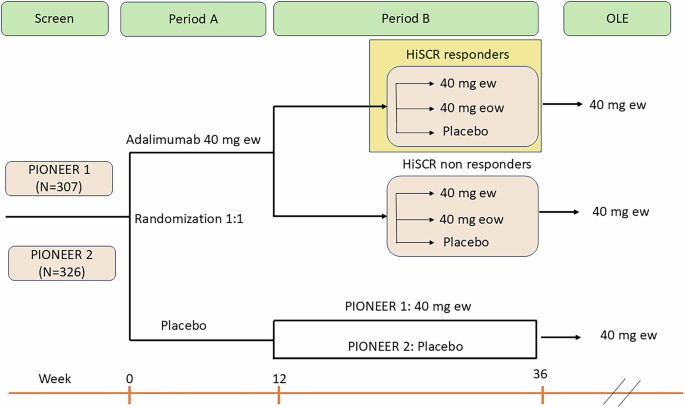
The analysis under discussion encompasses a select population of 199 patients, all of whom were re-randomized in a second phase (Period B) post an initial 12-week treatment of weekly adalimumab dosing. This re-randomization led to patients continuing either on adalimumab or switching to a placebo. Graphical representation of this setup is delineated in Figure 1, outlining the specific treatment assignments during the trial.
In terms of demographics, the studies showed a notable representation of female patients: 63.8% in PIONEER I and 67.8% in PIONEER II. The mean age of participants was approximately 37 years for PIONEER I and 35.5 years for PIONEER II, indicating a relatively young demographic undergoing treatment for HS.
Key Biomarkers Considered
The studies meticulously incorporated a series of biomarkers that hold clinical relevance. These biomarkers include:
- Abscess/nodule counts at baseline and after treatment,
- Fistula counts both initial and post-12 weeks,
- Reduction percentages in abscess counts,
- Current smoking status,
- Hurley stage at the start of the trial,
- Initial responder status based on the Hidradenitis Suppurativa Clinical Response (HiSCR).
Capturing these factors allows researchers to evaluate not only treatment efficacy but also the intricate relationships between these metrics and patient outcomes.
Ethical Oversight and Participant Consent
Both PIONEER trials adhere strictly to ethical guidelines established by the International Conference on Harmonisation and the Declaration of Helsinki. Study protocols were meticulously developed in collaboration with independent ethical committees at each participating site, ensuring that patient safety and data integrity were upheld. Importantly, informed consent was diligently obtained, allowing for secondary analyses on the collected data.
Subgroup Identification and Biomarker Analysis
The trials delve into an advanced framework for identifying subgroups of patients that could benefit optimally from the treatment offered. This is achieved through an observational data set denoted as {(Xi, Ai, Yi), i=1, …, n}, where i identifies individual patients, and outcomes are analyzed within a Neyman-Rubin potential outcomes framework.
The overarching goal in employing sophisticated statistical techniques is to derive individualized treatment rules (ITRs) that tailor treatment based on specific biomarker characteristics. In this way, the model can achieve precision in predicting who will respond best to adalimumab versus those who might fare better with alternative strategies.
DeepRAB Model: An Innovation in Prediction
Among the exciting advancements in the analysis is the introduction of DeepRAB, a deep neural network (DNN) model specifically tailored for estimating ITRs and determining significant predictive biomarkers. DeepRAB employs a three-component structure to facilitate comprehensive treatment effect modeling:
- A-learning approach for optimizing the loss;
- Biomarker selection layer for determining impactful predictors, leveraging methodologies akin to those found in Concrete Autoencoders;
- Multi-layer perceptron (MLP) which focuses on identifying and modeling non-linear relationships among covariates.
DeepRAB’s capability to efficiently manage high-dimensional data while selecting clinically relevant biomarkers positions it as a transformative tool in personalized medicine.
Simulation Framework and Scenarios
To robustly evaluate treatment effects, the researchers engaged in multiple simulation frameworks, considering varying complexities such as:
- Linear and nonlinear relationships between variables
- Interactive terms influencing treatment outcomes
These simulations bolster the validity of findings and enhance confidence in the predictive capabilities of the models employed.
Model Evaluation and Performance Metrics
A variety of baseline models were utilized, including causal forests and modified linear regression models, to facilitate a rounded exploration of treatment effects. The evaluation process emphasized the area under the ROC curve (AUC) for measuring model performance, which is crucial for establishing effective patient stratification. Notably, the model evaluation hinged on rigorous cross-validation strategies to ensure generalizability and to mitigate the risk of overfitting—a common concern in clinical research with potentially limited sample sizes.
Conclusion on Reporting and Implications
This detailed exploration of the cohort data from the PIONEER trials sheds light on the inherent complexities and considerations involved in treatment selection for HS. Each patient’s journey through this intricate landscape reveals the potential for precision medicine, where understanding the interplay of biomarkers and treatment responses could lead to transformative outcomes in clinical practice. Continuous refinement of models like DeepRAB will undoubtedly pave the way for a more tailored approach in managing hidradenitis suppurativa, emphasizing the necessity of leveraging data-driven insights to optimize patient care.