Exploring Spot Detection Methods in Fluorescence Microscopy: A Key to Advancing Biomedical Science
Introduction to Fluorescence Microscopy
Fluorescence microscopy stands as a cornerstone technique in biomedical imaging, enabling researchers to visualize cellular processes at unprecedented resolutions. The technology harnesses fluorescent dyes to label various cellular components, illuminating intricate biological mechanisms. However, the success of fluorescence microscopy hinges significantly on accurate spot detection methods, which are essential for quantifying fluorescent signals and deriving meaningful biological insights.
Understanding Spot Detection Challenges
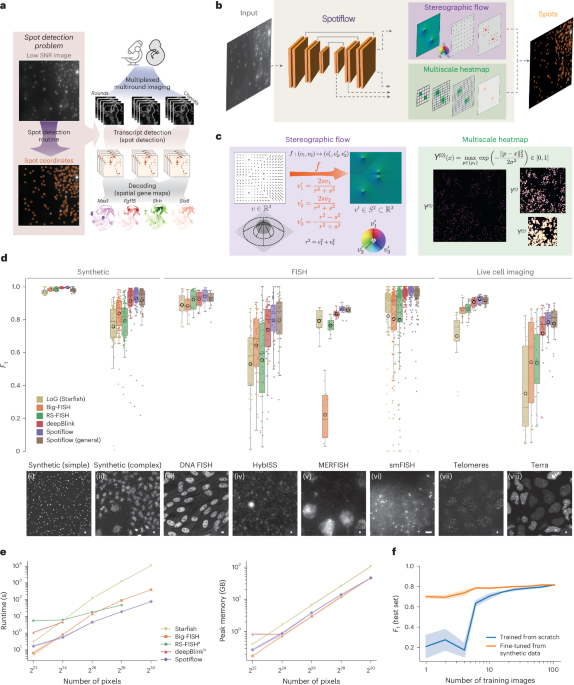
Spot detection refers to identifying and quantifying individual fluorescent signals—often representing molecules, proteins, or other cellular components—from an underlying noisy background. The challenge lies in differentiating these genuine signals from artifacts caused by inherent imaging inconsistencies, such as noise, overlap, and varying fluorescent intensities. As such, the quest for improving spot detection algorithms has become paramount to enhancing the reliability and interpretability of fluorescence microscopy data.
Quantitative Comparison of Detection Methods
A foundational study by Smal et al. (2010) critically analyzed various spot detection methods, offering a quantitative framework for comparing their performance in fluorescence microscopy. Their research highlighted key algorithms, including Gaussian fitting, wavelet-based detection, and local intensity maxima, emphasizing the importance of selecting the appropriate method based on the specific experimental scenario. The findings underscore that no single method universally excels; rather, effectiveness often relies on the nature of the data being analyzed.
Advancements in 3D Spot Detection
As fluorescence microscopy evolves, the need for three-dimensional (3D) imaging has grown. A study by Štěpka et al. (2015) pursued sensitivity evaluations of 3D spot detection methods in confocal microscopy. This research demonstrated that while traditional 2D methods provide valuable insights, the transition into the third dimension significantly enriches spatial understanding of cellular structures and dynamics. Subsequent 3D methods combine algorithms that effectively capture complex spatial relationships, revealing the intricate architecture of cells.
The Role of Super-Resolution Techniques
Super-resolution microscopy has revolutionized our capabilities by allowing researchers to visualize structures at resolutions surpassing diffraction limits. Sage et al. (2019) assessed various software for 2D and 3D single-molecule localization microscopy, emphasizing the trade-offs between detection speed and accuracy. The results indicated that while advanced algorithms yield impressive spatial resolutions, computational demands necessitated the development of efficient software pipelines for real-time applications in live-cell imaging.
The Power of Automated Image Analysis
Automating detection processes is not merely a convenience but a necessity, particularly in high-throughput studies where vast quantities of data are generated. Steinfath et al. (2001) introduced automated image analysis techniques tailored for hybridization experiments. Their methodologies exploit computational frameworks that minimize human error and enhance reproducibility, thereby setting a precedent for subsequent research geared towards automated feature extraction in microscopy images.
Bioinformatics Integration
Bioinformatics plays a critical role in optimizing image analysis workflows. Dowsey et al. (2003) explored how integrating bioinformatics into two-dimensional gel electrophoresis significantly enhances the analysis of protein expression. Similarly, modern fluorescence microscopy benefits immensely from bioinformatics approaches, streamlining data processing, and enhancing the interpretability of complex datasets.
Spotlight on Recent Innovations
Emerging tools in spot detection, such as deep learning-based methodologies, promise to transform the landscape of fluorescence microscopy. For instance, the introduction of algorithms like deepBlink and SpotLearn has demonstrated exceptional proficiency in detecting fluorescence in situ hybridization (FISH) signals, showcasing how convolutional neural networks can optimize detection accuracy and speed in high-throughput settings. Research by Eichenberger et al. (2020) highlights the efficacy of these advanced techniques, suggesting their potential for widespread application across diverse biological research domains.
Overcoming Autofluorescence Challenges
Autofluorescence remains a significant obstacle to accurate imaging in biological samples. Yang et al. (2017) addressed this concern by investigating methods for quenching autofluorescence in tissue specimens during immunofluorescence protocols. Their findings revealed strategic approaches that can markedly improve signal clarity, thereby facilitating more accurate data interpretation.
Multiplexing Techniques
Recent advancements have rendered it possible to conduct multiplexed imaging, significantly enriching the data acquired from individual samples. Choi et al. (2018) introduced third-generation in situ hybridization chain reaction techniques that allow for simultaneous detection of multiple RNA species within the same sample, opening avenues for studying spatial distribution and interaction of biomolecules in tissue structures.
The Evolution of Software and Tools
The software landscape for image analysis is rapidly expanding, with tools like Fiji and BigStitcher exemplifying how user-friendly platforms can support complex analysis tasks. This evolution aligns with the need for accessible yet powerful computational tools that cater to researchers at varying levels of expertise within the field.
Future Directions
As technology progresses, the trajectory of spot detection methods in fluorescence microscopy promises even more sophisticated tools and methods. Increased collaboration between computational scientists and biologists will foster innovations that enhance our capacity for real-time, high-resolution imaging. The ultimate goal remains clear: to leverage these advancements in understanding dynamic biological processes fundamentally and to drive forward the frontiers of biomedical research.
In this detailed exploration of spot detection in fluorescence microscopy, we have illustrated the critical importance of understanding and improving detection methods. Each section reflects the continuous journey towards enhancing imaging techniques, directly influencing our comprehension of cellular biology and pathology. Whether analyzing intricate protein interactions or assessing spatial gene expression, the tools and methodologies discussed represent the cutting edge of microscopy research.