Revolutionizing Pancreatic Cyst Diagnosis: A Multicenter Validation of a Deep Learning CT Model
Revolutionizing Pancreatic Cyst Diagnosis: A Multicenter Validation of a Deep Learning CT Model
A recent multicenter study has introduced a groundbreaking deep learning model to enhance the diagnosis of pancreatic cystic neoplasms (PCNs). This model serves not only to improve diagnostic accuracy but also has the potential to significantly reduce the workloads of radiologists, offering tangible benefits in clinical settings.
Importance of Pancreatic Cyst Diagnosis
Pancreatic cystic neoplasms encompass various types of cysts that can range from benign to pre-cancerous and malignant. Accurate diagnosis is critical, as misdiagnosis could lead to inappropriate management strategies and potential patient harm. The deep learning CT model developed in this study aims to streamline this process and enhance diagnostic precision, thereby minimizing the risk of over-treatment and ensuring timely interventions for patients at risk.
Key Components of the Deep Learning Model
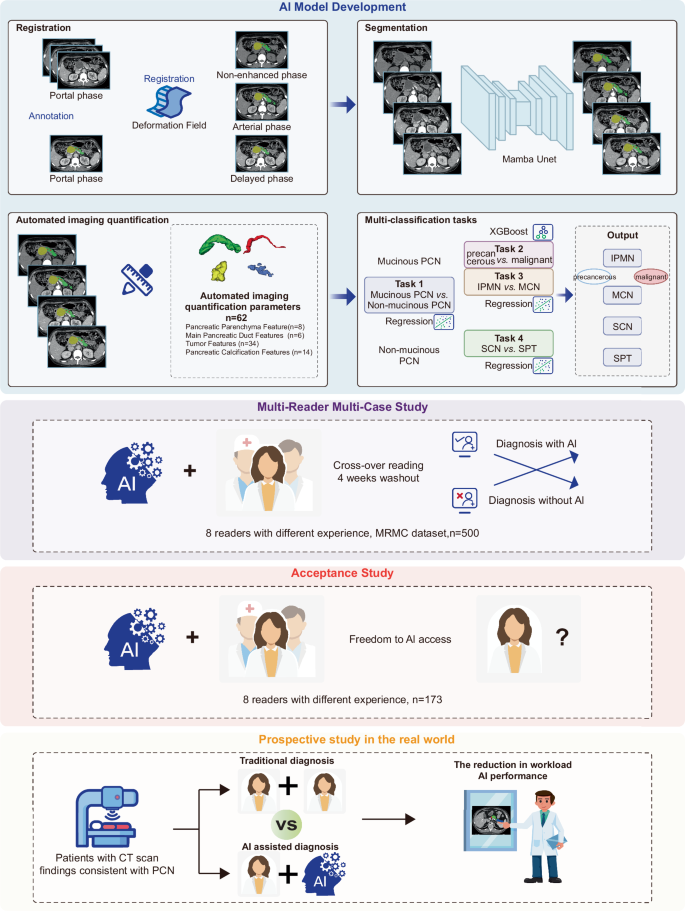
The study incorporated several pivotal components that distinguish its approach:
-
Data Collection: Roughly 2,359 patients with PCNs were recruited from four hospitals over 11 years. Ultimately, 1,835 patients met the inclusion criteria for model training.
-
Image Segmentation: Image segmentation is a process where the deep learning model identifies different structures within images. Using segmentation models, the study delineated key CT characteristics relevant to cyst classification.
- Classification Tasks: The model underwent four classification tasks, each targeting distinct types of cysts—mucinous vs. non-mucinous cysts and further sub-classifications of mucinous cysts into precancerous and malignant categories.
The effective integration of these components showcases how advanced algorithms can comprehensively analyze and interpret complex medical imaging data.
Step-by-Step Process of Model Development
The model development process unfolded in four major stages:
-
Stage One – Image Segmentation: The initial focus was on training a model to extract CT imaging features. Each feature helps to assess the malignancy potential based on the segmented images.
-
Stage Two – Classification Tasks: In this stage, the model performed its classification tasks based on previously collected features. Each task was designed to parse the intricacies of PCNs and categorize them accurately.
-
Stage Three – Multi-Reader Study: A diverse group of eight readers, with varying levels of experience, participated to validate the AI model against traditional diagnostic approaches. This stage evaluated diagnostic accuracy enhancements post-AI assistance.
- Stage Four – Real-World Data Cohort: This phase tested the model’s effectiveness in a real clinical setting, comparing it against standard double-reader methodologies. Such validation is crucial as it assesses the model’s practical applicability in everyday diagnostic workflows.
Practical Examples and Case Studies
In one practical scenario described in the study, the AI model effectively classified 1,249 patients between mucinous and non-mucinous cysts. Using an area under the curve (AUC) metric, the model recorded an AUC of 0.946 for the training set and 0.942 for the validation set, indicating remarkable reliability in its predictive capabilities.
Furthermore, in real-world implementations, the AI-assisted diagnosis improved radiologist performance significantly. For instance, average diagnostic times were reduced from 5.28 minutes to 4.03 minutes when using AI, demonstrating not only improved accuracy but also efficiency.
Common Pitfalls and How to Avoid Them
Even with promising results, the study identified potential pitfalls:
-
Data Imbalance: There was a marked imbalance in the number of benign, malignant, and pre-cancerous cases within the datasets. This can lead to biases in the model’s predictions. Addressing data imbalance through techniques like oversampling or synthetic data generation can enhance model reliability.
- Overfitting: Some models showed signs of overfitting, particularly those that performed well on training data but poorly on validation datasets. Techniques such as cross-validation and regularization can mitigate this issue.
Tools and Metrics in Use
The evaluation of the deep learning model utilized several statistical tools, including:
-
Receiver Operating Characteristic (ROC) Curves: These curves illustrate the diagnostic ability of the model across various threshold settings. Their insights provide a clear view of trade-offs between sensitivity and specificity.
- Area Under the Curve (AUC): AUC is a crucial metric here, as it serves as a singular measure summarizing the model’s performance. Higher AUC values indicate better model performance.
Radiologists and researchers are already seeing the benefits of these tools, as evidenced by improved diagnostic accuracy metrics tied to AI-enhanced workflows.
Variations and Alternatives
While the deep learning model presented in this study offers stellar performance, alternatives do exist. For example, traditional imaging techniques still play vital roles in diagnostic processes, especially for cases where advances in AI have not yet penetrated. While deep learning models excel in detail and pattern recognition, they require substantial training data and computational resources.
FAQs
-
How does the AI model differentiate between types of cysts?
The model utilizes quantitative imaging features derived from CT scans to distinguish between mucinous and non-mucinous cysts, employing complex algorithms that analyze these features for patterns indicative of malignancy. -
What is the significance of segmentation in this model?
Segmentation allows the model to isolate specific anatomical structures within CT images, improving its ability to analyze features that may indicate cancer risk. -
Will the model replace human radiologists?
No, the model is intended to assist radiologists, enhancing their diagnostic capabilities and efficiency rather than replacing the critical role that human expertise plays in medical diagnosis. - What future improvements are anticipated for this technology?
As AI technology continues to evolve, further refinements in model accuracy and the integration of larger datasets from diverse populations will enhance the reliability and robustness of these diagnostic tools.