Quantum Kernel Drug-Target Interaction Prediction: A Revolutionary Approach
Introduction to QKDTI
The rise of computational techniques in drug discovery has ushered in groundbreaking methodologies, and one notable development is the Quantum Kernel Drug-Target Interaction (QKDTI) framework. This innovative quantum-enhanced regression model leverages the principles of quantum computing to elevate the accuracy and efficiency of predicting drug-target interactions (DTIs). Traditional methods have often struggled with the complexities inherent in high-dimensional feature spaces and nonlinear molecular interactions. However, QKDTI stands to redefine the paradigms of DTI prediction through its unique capabilities.
The Challenge: Traditional Approaches
Current classical machine learning (ML) and deep learning (DL) techniques face significant hurdles in accurately modeling drug-target interactions. These methods, including Support Vector Regression (SVR), often rely on kernel functions like linear, polynomial, and radial basis functions (RBF) to map complex data into higher-dimensional spaces. Although effective in some scenarios, these traditional SVR kernels frequently fall short when confronted with the intricate nonlinear relationships produced by molecular structures.
QKDTI: A Quantum Leap Forward
QKDTI offers a transformative alternative through the utilization of quantum kernels, which efficiently map complex molecular interactions into higher-dimensional Hilbert spaces. Unlike classical kernels, quantum kernels exploit quantum superposition and entanglement, allowing for far richer representations of protein-ligand interactions. This mechanism not only aids in improving the precision of drug-target binding affinity predictions but also enhances the ability to navigate the complexities of molecular structures.
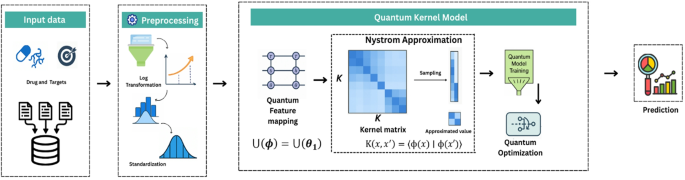
Methodology Overview
The framework comprises several systematic steps to ensure optimal performance, predominantly involving quantum-enhanced feature transformations, coupled with traditional quantum machine learning techniques.
-
Dataset Acquisition
Leveraging datasets from the Therapeutics Data Commons, three extensively validated datasets—DAVIS, KIBA, and BindingDB—are employed for training, testing, and validation of the model. These datasets encompass diverse drug-target pairs and their corresponding binding affinities drawn from rigorous experimental metrics. - Molecular Representations
Molecular data is encapsulated using various representation methods:- Small Molecules are encoded through SMILES (Simplified Molecular Input Line Entry System) strings.
- Proteins are represented via FASTA sequences, reflecting their unique amino acid sequences.
When preprocessing data, molecular fingerprints are derived using cheminformatics tools like RDKit, while protein features are extracted via pre-trained models, ensuring a comprehensive representation suitable for machine learning applications.
Data Preparation and Standardization
Robust preprocessing is critical in any predictive modeling framework. QKDTI adopts several preparatory steps to standardize inputs and ensure a consistent training environment:
- Log Transformation: Binding affinity measurements undergo logarithmic transformation to stabilize variance and normalize the broad range of raw values, enhancing model performance.
- Z-score Normalization: Features are standardized to have a mean of zero and unit variance, preventing any single feature from disproportionately influencing predictions.
- Dimensionality Reduction: Principal Component Analysis (PCA) is applied to retain essential features while expelling redundant molecular descriptors, optimizing the quantum embedding process.
Quantum Feature Mapping
After establishing a solid preprocessing pipeline, the model moves into the quantum feature mapping stage. At this point, molecular descriptors are encoded into a quantum Hilbert space via parameterized quantum circuits (PQC). The use of quantum circuits allows for complex interactions among molecular features by placing qubits in superposition, thus enhancing the model’s generalization capabilities.
- Hadamard Gates: Each qubit is initialized using Hadamard gates, facilitating quantum parallelism, which is vital for efficient feature representation.
- Parameterized Rotation Gates: Successive transformations of each qubit ensure that the model captures intricate dependencies among molecular features through controlled operations.
Efficient Computational Strategies
Given the exponential growth of computation complexity associated with quantum kernels, QKDTI employs a Nyström approximation. This technique streamlines kernel evaluations by using a smaller subset of data, retaining high accuracy while mitigating computational expenses.
Quantum Optimization Framework
Once quantum kernels are computed, they interface with the QKDTI model, which follows a familiar epsilon-Support Vector Regression (SVR) optimization framework. The focus here is on minimizing loss functions while managing complexity and ensuring high predictive accuracy.
Hyperparameter Optimization
In optimizing the QKDTI model, fine-tuning of hyperparameters is a crucial pursuit. The critical parameters include:
- Quantum Circuit Parameters: Managing the number of qubits and the types of gates utilized.
- Kernel Approximation Parameters: Adjusting batch sizes in the Nyström approximation to enhance computational efficiency.
- SVR Parameters: Determining the regularization parameter and defining tolerances for predictions to strike a balance between accuracy and generalization.
Computational Environment
The QKDTI model’s implementation is harmonized through a hybrid quantum-classical computational framework, utilizing tools such as PennyLane for quantum machine learning, Qiskit for quantum backend operations, and classical ML libraries, including Scikit-learn, PyTorch, and TensorFlow. This multi-faceted computational approach ensures performance efficiency and scalability for all predictive modeling tasks.
Final Thoughts
The QKDTI framework represents a substantial advancement in the field of drug discovery, harnessing the capabilities of quantum computing to provide accurate predictions of drug-target interactions. By overcoming the limitations of traditional methods and employing a robust quantum-enhanced approach, QKDTI stands at the forefront of computational drug design, paving the way for more efficient and precise medicine development in the future.