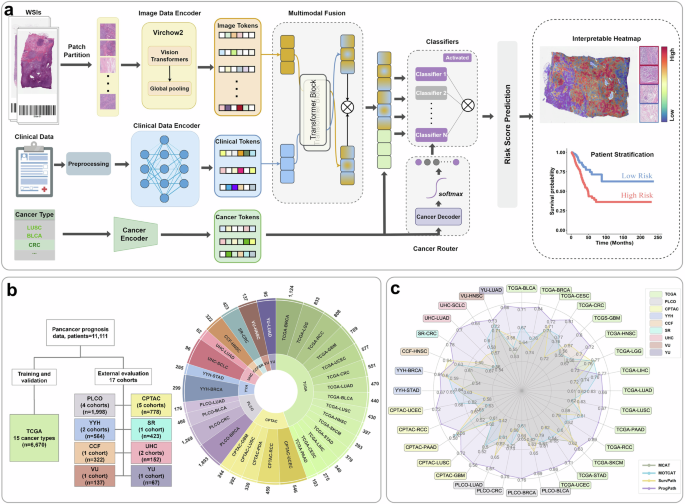
Overview Architecture of PROGPATH: A Unified Model for PanCancer Survival Prediction
Introduction to PROGPATH
The architecture of PROGPATH represents a groundbreaking leap in the realm of cancer prognosis, unifying various data inputs to create a comprehensive survival prediction model. As illustrated in the accompanying figures (Fig. 1), this model utilizes advanced computational techniques to analyze both histopathological images and clinical variables, producing meticulous patient-level survival predictions.
Image Preprocessing Techniques
At the core of PROGPATH, standardized image preprocessing techniques play a crucial role. Initially, we employ patch-level feature extraction from digitized hematoxylin and eosin (H&E) slides using Virchow2, a Vision Transformer-based foundation model. This methodology underscores the significance of tiling, allowing us to dissect whole slide images (WSIs) into smaller, analyzable patches. The extracted features are the building blocks necessary for further analysis, establishing a strong foundation for the prediction model.
Integration of Clinical and Histopathological Features
The next phase in the PROGPATH workflow involves the aggregation of extracted image features with clinical and cancer-type data. This fusion occurs through a cross-attention transformer module, which effectively models intermodal interactions. Clinical variables such as age, sex, and tumor staging are embedded through independent fully connected networks, creating a robust combined feature set. This innovative approach allows for a nuanced understanding of how histopathological insights correlate with clinical indicators, enhancing the model’s predictive accuracy.
The Cancer-Aware Router
An innovative element of the PROGPATH architecture is the cancer-aware router, which dynamically selects domain-specific classifiers based on the cancer type involved. This tailored approach ensures that survival predictions are not only accurate but also specific to individual cancer forms. The integration of this router further emphasizes how PROGPATH moves beyond generalized models, adapting to the heterogeneous nature of cancer.
Training and Evaluation Using Extensive Datasets
Model training for PROGPATH was conducted using the TCGA cohorts, encompassing a substantial 7999 WSIs from 6670 patients across 15 different cancer types. A rigorous five-fold cross-validation approach was employed, promoting a robust understanding of model performance. This groundwork was essential for evaluating the generalizability of PROGPATH through extensive external validation on 17 independent cohorts, incorporating diverse clinical settings and patient demographics.
Performance Metrics and Comparisons
To evaluate the efficacy of PROGPATH, survival outcomes were defined based on reliable endpoint annotations. Our results, detailed in the figures, reflect significant improvements in the concordance index (C-index), where PROGPATH notably surpassed conventional models, including Cox proportional hazards models and other multimodal survival prediction models. This comprehensive evaluation underscores the potential of PROGPATH to refine prognostic approaches in clinical settings.
Performance Across Cancer Types
In a comparative analysis against recent state-of-the-art models such as MCAT, MOTCAT, and SurvPath, PROGPATH consistently demonstrated its superiority across multiple cancer types. This broader performance evaluation, grounded in both in-distribution and out-of-distribution datasets, illustrates the robustness and generalizability of the PROGPATH model.
Robustness in Stratification Across Cancer Stages
Understanding the clinical implications of cancer staging is critical for treatment planning. PROGPATH’s capability to stratify patient outcomes across different cancer stages—early and advanced—further highlights its detailed prognostic insights. The model’s ability to discern risk levels among patients with early-stage cancers reflects its potential to significantly impact treatment decisions and patient management strategies.
Treatment-Specific Predictions
To ensure that PROGPATH captures variations in patient survival outcomes based on treatment types, we examined its predictive performance within subgroups defined by pharmacological or radiotherapeutic interventions. The distinguishing capability of PROGPATH in stratifying patients into risk categories emphasizes its clinical relevance and potential utility in personalized medicine approaches.
Biomarker-Defined Subgroup Analysis
In the era of precision oncology, the importance of genomic biomarkers cannot be understated. PROGPATH enhances prognostic assessments within biomarker-defined subgroups as exemplified by our analyses focusing on key cancer biomarkers. The model’s proficiency in stratifying patients based on biomarker status underscores its ability to provide truly tailored prognostic insights.
Conclusion
Through its innovative architecture and robust data integration strategies, PROGPATH stands at the forefront of cancer prognosis modeling, promising a future where personalized treatment planning and improved patient outcomes are achievable through actionable insights derived from complex cancer datasets.