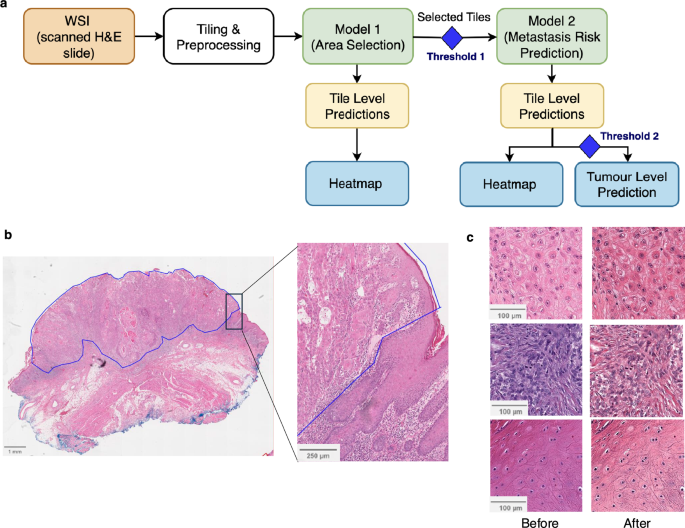
Overview of cSCCNet
cSCCNet represents a significant advancement in the automated assessment of cutaneous squamous cell carcinoma (cSCC). This innovative system encompasses two primary models: Model 1, which specializes in ‘automated area selection,’ and Model 2, designed for ‘prediction of metastatic risk.’ These models work hand-in-hand to enhance diagnostic accuracy and improve patient outcomes.
Model 1: Automated Area Selection
The first step within the cSCCNet framework is the automated selection of regions of interest (ROI) from whole slide images (WSI). Given that WSIs often contain non-tumor artifacts and normal tissue, the precision of this selection is paramount. Model 1 hones in on specific areas defined as tumor, intratumoral inflammatory cells, and peri-tumoral stroma—components identified as critical in tumor progression.
Once the ROI is established, tiles are extracted from these regions. These tiles serve as the input for Model 2, which assesses the risk of metastasis for the tumor in question. This tile-level prediction first identifies high-risk and low-risk tiles, effectively filtering out noise from the data and ensuring that only the most relevant information is considered for the final tumor-level assessment.
Developing Model 1
To craft Model 1, researchers compiled WSIs from a diverse cohort of 227 cSCC cases across four different centers. Each WSI was meticulously annotated by an expert dermatopathologist to pinpoint the crucial ROI. This vetting process resulted in the extraction of over 460,000 tiles, which were categorized into ROI and non-ROI segments.
Color normalization was a vital preprocessing step, enhancing image consistency across varying samples. The model was trained on a major portion of the ROI tiles using KerasTuner to compare different deep learning architectures. The optimal model, based on the ResNet50 architecture, exhibited an impressive tile-level accuracy of over 90%, demonstrating robust performance across multiple validation folds.
In testing, Model 1 achieved an area under the curve (AUC) of 0.97, indicating an exceptional ability to identify ROI in comparison to pathologist annotations.
Model 2: Prediction of Metastatic Risk
The second model, Model 2, steps into the arena of predicting whether the cSCC samples may metastasize. It utilizes the tiles identified as ROI by Model 1 for in-depth analysis.
Developing Model 2
Model 2 was trained on a substantial dataset of 129,187 ROI tiles classified from samples that either did or did not metastasize. To prevent bias towards larger tumors, only 500 tiles were randomly selected per tumor, leading to a balance of 27,920 tiles from metastasizing tumors and 32,711 tiles from non-metastasizing ones.
Similar to Model 1, this model underwent rigorous training phases using the ResNet50 architecture and was advanced through five-fold cross-validation. The model exhibited a mean tile-level accuracy of 0.92 for training and 0.76 for validation. Impressively, when both models were utilized sequentially, cSCCNet achieved an outstanding 98% accuracy in predicting which tumors metastasized.
Performance Metrics and Evaluation
In its performance evaluation, cSCCNet was rigorously tested against a cohort of 40 cSCC samples. Using predefined thresholds for Model 1 (≥0.65) and Model 2 (median score >0.2), the system was able to classify 38 out of 40 cases correctly, showcasing a superior AUC of 0.95 compared to existing models.
The testing also revealed that cSCCNet significantly outperformed traditional clinicopathologic classifications, reflecting not only its diagnostic potency but also its potential utility in clinical settings.
Cross-Center Variability and Generalizability
Understanding the variability across different centers is crucial for model robustness. A center-split cross-validation approach was employed, wherein a model trained on data from three centers was tested using data from a fourth, unseen center. Although performance was somewhat diminished, the model retained reasonable predictive capabilities, underscoring the necessity of diverse data during training to enhance generalizability.
Histopathology and Model Explainability
To bolster the explainability of cSCCNet, histopathological reviews were conducted alongside multiplex immunohistochemistry (IHC) analyses on select metastasizing and non-metastasizing cases. Preliminary observations indicated that certain histopathological features, such as poorly differentiated carcinoma or specific inflammatory signatures, often correlated with high-risk scores, providing valuable insights into model predictions.
High scores inflected areas characterized by invasive behavior or necrosis, whereas low scores were associated with well-differentiated tumors. This understanding aids in bridging the gap between computational predictions and traditional histopathological insights.
Reviewing Model Limitations
Despite the many successes of cSCCNet, two cases within the testing cohort were misclassified. One non-metastasizing cSCC received a high score due to histopathological characteristics that posed challenges for the model. Conversely, a metastasizing cSCC was assigned a low score, attributed to a lack of sufficient tiles representing its aggressive nature. These instances highlight the ongoing need for refinement and adaptability in predictive models to improve accuracy further.