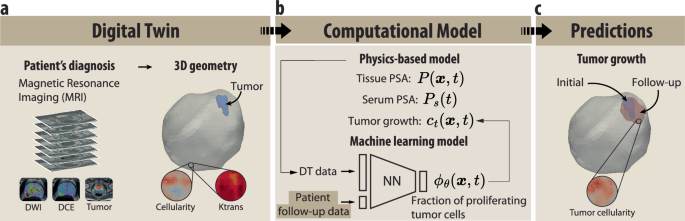
Physics-Informed Machine Learning Digital Twin Framework for Prostate Cancer
Prostate cancer is one of the most common cancers among men, and its management often requires an individualized approach. In recent years, a groundbreaking computational paradigm has emerged, merging physics-based modeling with machine learning: the digital twin framework. This innovative strategy allows for real-time simulations of tumor growth, providing a patient-specific interactive model that may revolutionize the way clinicians approach prostate cancer treatment.
Understanding the Digital Twin Framework
At the heart of this framework lies a marriage between two substantial components: a physics-based model and a machine-learning (ML) model. This framework uses T2-weighted MRI sequences, including Diffusion Weighted (DW) and Dynamic Contrast Enhanced (DCE) images, along with tumor segmentations provided by expert radiologists to create a detailed 3D representation of a patient’s prostate.
Imaging and Data Extraction
The digital twin is generated from these sophisticated MRI sequences, leading to a voxelized 3D geometry that captures critical patient data. The model includes various metrics, including cellularity ( c(x, t) ), vascularization ( k{\text{trans}}(x) ) to assess tissue perfusion and permeability, and a tumor binary mask ( T{\text{mask}}(x, t) ). Each of these components contributes essential information about the tumor’s characteristics and behavior, allowing for accurate simulations of tumor dynamics.
Figure 1: Prostate cancer digital twin framework.
Physics-Based Modeling of Tumor Growth Dynamics
The physics-based model acts as a fundamental engine, simulating various biological processes involved in prostate cancer progression. A significant aspect is the modeling of prostate-specific antigen (PSA) dynamics, which reflects the leakage from cancer cells to the blood.
The model accounts for:
- PSA Exchange: It incorporates the exchange of tissue PSA and serum PSA, depending on the concentration gradient and capillary permeability.
- Tumor Growth: The concentration of tumor cells ( c_t(x, t) ) is critical, as it informs the production of tissue PSA, thus linking tumor dynamics with serum PSA variations.
This model effectively captures the biological mechanisms of tumor growth, allowing healthcare practitioners to predict how the disease might evolve over time based on initial diagnostic imaging.
Integrating Machine Learning for Enhanced Predictions
To streamline the prediction of tumor dynamics, an ML model supplements the physics-based framework. This deep-learning algorithm approximates the fraction of proliferating tumor cells ( \phi_{\theta}(x, t) ) and regulates growth dynamics using historical serum PSA test data from the patient.
Neural Network Configuration
The neural network (NN) receives an array of inputs, including:
- Cellularity and Normalized PSA Levels: Both tissue-specific and serum PSA levels are included, ensuring a comprehensive dataset for accurate predictions.
- Neighboring Voxels Data: By considering not only the voxel of interest but also its surrounding context, the model captures the spatial dynamics of tumor growth.
This integration enhances the model’s capacity to adapt and refine predictions based on real-time data, thereby personalizing the treatment strategy.
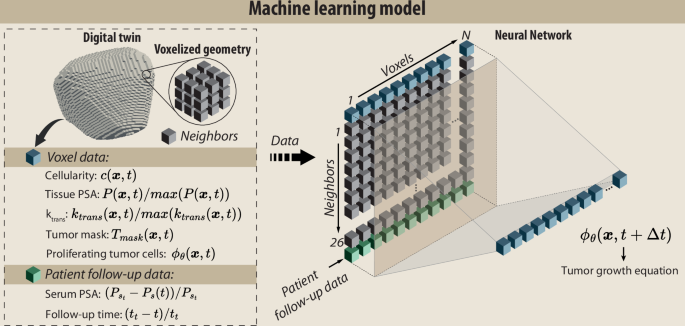
Figure 2: Machine learning model.
Calibration: Tailoring Predictions to Individual Patients
Parameter calibration is vital for the effectiveness of the digital twin framework. The process takes place in two phases.
- Training the General NN: The model is initially trained on a broad dataset to grasp general PSA dynamics, informed by biological processes and tumor growth factors.
- Patient-Specific Calibration: With a single set of MRI data related to a particular patient, the NN is fine-tuned to optimize the physics-based model parameters. This calibration ensures that the tumor predictions accurately reflect the individual patient’s tumor behavior.
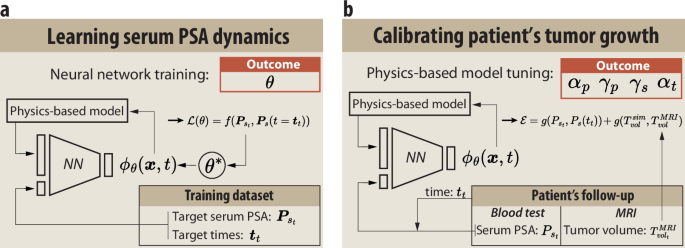
Figure 3: Calibration of the framework to reproduce patient-specific tumor growth.
Insights from Patient Data: A Comparative Analysis
The potential of this digital twin framework is illustrated through anonymized data from two patients, referred to as Patient A and Patient B. Despite similar Gleason scores, their tumor characteristics demonstrated considerable variability.
- Patient A presented a lower PSA production rate but showed a significantly higher tumor growth rate compared to Patient B.
- Model Accuracy: The calibrated models accurately predicted tumor growth dynamics and serum PSA levels, with only minor discrepancies observed during follow-ups.
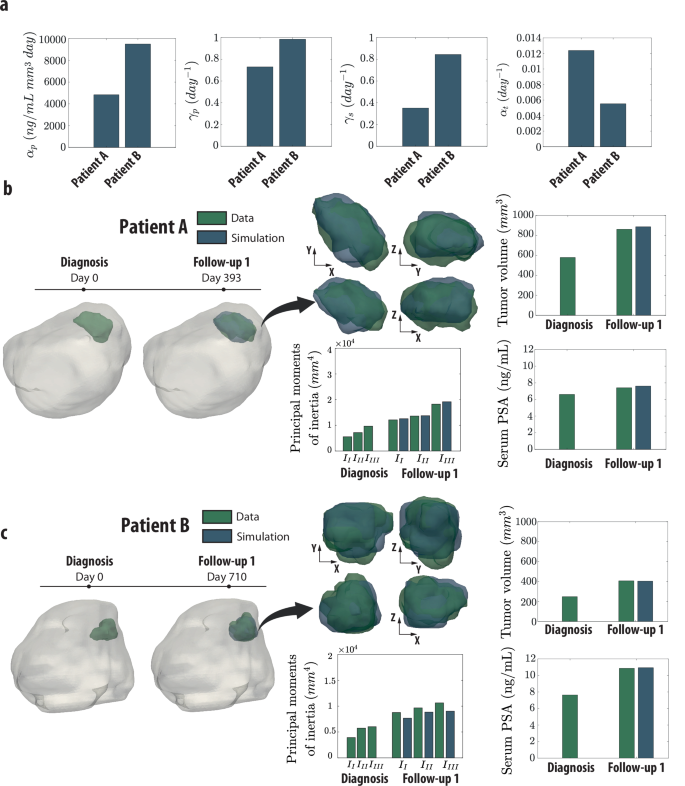
Figure 4: Patient-specific prostate tumor growth.
Long-Term Growth Prediction from PSA Measurements
Arguably one of the most compelling features of the digital twin framework is its capability to predict long-term tumor growth based solely on serum PSA levels collected during follow-ups, potentially mitigating the need for frequent MRIs.
Continued Monitoring
Using established physics-based model parameters tied to individual patients, future tumor growth can be inferred exclusively from serum PSA data. This strategy emphasizes the significance of ease of monitoring, allowing healthcare providers to maintain close observation of patient conditions without invasive procedures.
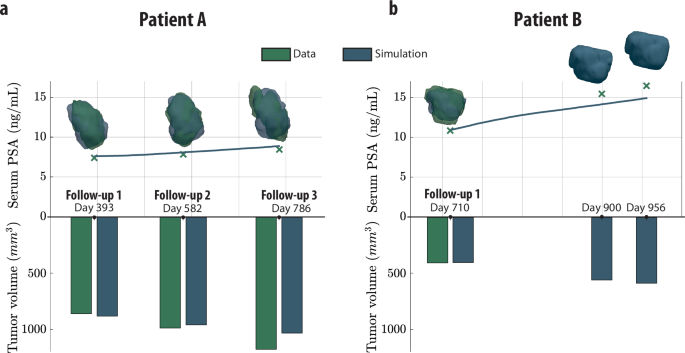
Figure 5: Reconstruction of patient-specific prostate tumor growth.
Understanding the intricacies of tumors and how they evolve poses significant challenges in oncology. However, the integration of machine learning with physics-informed methodologies provides a robust framework for personalized cancer treatment, enhancing the understanding and management of prostate cancer.