Unveiling the Multimodal Integration Classifier (MIC) for Ion and Water Labeling: An In-Depth Look
The Multimodal Integration Classifier (MIC) stands at the forefront of machine learning (ML) methodologies applied to structural biology, particularly in the classification of waters and ions within protein structures. Leveraging advanced approaches in data representation and classification, MIC addresses a critical need: accurately identifying the chemical microenvironments surrounding ions and water in protein structures derived from cryo-electron microscopy (cryo-EM) and X-ray crystallography.
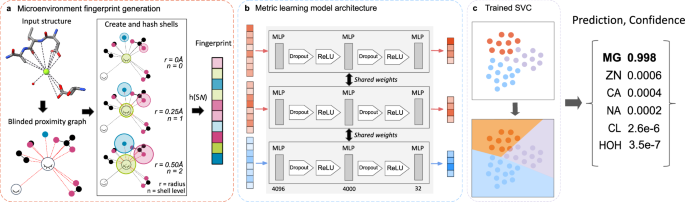
The MIC Workflow: Step-by-Step
The workflow of MIC is an elegant three-step process. It begins with generating a fingerprint representation of the chemical environment surrounding specific sites—typically ions or water. This involves constructing a proximal interaction graph containing all atoms within a 6 Å radius of the density of interest. By iteratively capturing local chemical information, MIC hashes atomic invariants and their respective interactions within successive shells surrounding each atom.
Next, these generated fingerprints undergo dimensionality reduction through a deep metric learning model. This model operates on a feed-forward network, condensing complex information into a more manageable 32-dimensional embedding. The final step employs a support vector classifier (SVC) to produce output probabilities for various classes, including ions and waters, accompanied by a measure of prediction confidence. This three-pronged methodology empowers MIC to build accurate models even when working with relatively small datasets.
Overcoming Challenges of Traditional Methods
Traditional approaches to classifying ion and water sites have often relied on voxel representations fed into complex neural networks. Such architectures are not only orientation-dependent but also require extensive training data to avoid overfitting. MIC counters these challenges by utilizing data filtering techniques critical for ensuring high-quality training. The fingerprint model significantly reduces the dimensional complexity of its representations, adeptly capturing crucial chemical interactions while sidestepping the pitfalls of abundant data requirements.
Performance Metrics: An Impressive Track Record
In its evaluations, MIC has demonstrated impressive performance. Training on various prevalent classes from the Protein Data Bank, the model achieved an initial accuracy of 78.6% on a held-out test set. Performance trends were particularly favorable for chemically significant ions like zinc, magnesium, calcium, and water, with accuracy rates rising for these categories. This success is partially attributed to the model’s ability to learn and visualize embedding spaces that reflect the chemical charge of ions, revealing intrinsic relationships without pre-encoding such information in the training.
Insight into the Learned Embedding Space
A standout aspect of MIC’s modeling approach is its ability to organize learned embeddings based on charge, visualized through techniques like uniform manifold approximation and projection (UMAP). This emergent quality provides insight into structural relationships within the dataset. Interestingly, MIC appears to maintain strong resilience against small variations (≤ 0.5Å) in ion positioning while consistently exhibiting confidence in its predictions.
Interpretability: Addressing the Black Box Issue
One of the significant obstacles within ML models is their interpretability—or lack thereof. MIC confronts this issue head-on through integrated gradient techniques, which quantify the contribution of each input feature to output predictions. This capability fosters a deeper understanding of what the model considers salient. Through pairwise feature attribution, researchers can identify key residues, interactions, and molecular features that contribute to differentiating between similar ion sites.
For instance, in comparing the fingerprints of zinc and magnesium, features such as sulfur from cysteine or carboxyl groups showed high attribution values, revealing the distinct interactions that influence classification. By assessing the importance of these features, MIC provides a robust biophysical rationale behind each prediction.
Manual Review of Discrepant Predictions
MANUAL REVIEW OF DISCREPANT PREDICTIONS adds a layer of scrutiny and refinement to the classification process. In a review of early predictions, discrepancies became apparent in predicted labels versus experimentally derived sites. A dedicated team reassessed 471 questionable examples and determined that MIC accurately labeled 142 of the initially misidentified sites. This manual inspection also spotlighted instances of incorrect annotations, often attributing the errors to atypical models, highlighting MIC’s potential resilience and adaptability in classification tasks.
Expanding the Boundaries: Testing on Cryo-EM and RNA Structures
The versatility of MIC extends beyond X-ray structures, entering the realm of cryo-EM where resolution varies significantly. Initial tests have shown promising results, with MIC effectively identifying ion sites under different resolutions. In evaluating bacterial ribosomal structures, for example, the model displayed commendable accuracy, correctly identifying over 90% of ion and water assignments. Furthermore, extending MIC’s capabilities to RNA structures proved fruitful, signaling potential in diverse biological systems where ion interactions play a pivotal role.
Growing the Ion Spectrum: Extended Set Model
Recognizing the limitations inherent in the prevalent ion model, MIC has developed an extended set model. This variant introduces underrepresented ions such as potassium, iron, and manganese. While training data remains limited, initial evaluations yielded an impressive accuracy of 79.1% on this newly expanded set. Following manual reviews, performance climbed to 86.5%, underscoring the importance of iterative refinement and thorough dataset management.
Comparative Analysis: MIC Versus Existing Tools
In a landscape dotted with numerous tools designed for similar tasks, MIC distinguishes itself through its precise labeling capabilities and confidence scoring. Notably, comparisons with tools like CheckMyMetal and CheckMyBlob revealed MIC’s superior accuracy in situations where ion classification was paramount. By focusing on generating distinct class predictions—with specific probabilities tied to ion identities—MIC facilitates rapid classifications that can directly guide further experimental validations.
Conclusion: Breaking New Ground in Structural Biology
MIC not only enhances the accuracy of ion and water labeling in structural biology but also provides an interpretative backbone that many existing methods lack. From its efficient workflow that circumvents typical data-heavy approaches to its capability for manual review and expansion to diverse classes, MIC serves as a transformative tool for researchers diving into the complexities of molecular interactions.
By seamlessly integrating computational intelligence with structural biology, MIC promises to usher in a new era of biophysical insights and applications, challenges notwithstanding. With ongoing enhancements and an established framework, the tool reflects the cutting-edge intersection of technology and biological sciences. The journey of discovering molecular intricacies, once laborious and laden with ambiguities, is being refined through the intelligent deployment of machine learning with MIC at its helm.