Subject Recruitment and Ethical Approval in a Clinical Trial for Parkinson’s Disease: A Detailed Overview
Introduction
Navigating the complexities of clinical research requires a meticulous approach to subject recruitment and ethical approval, particularly in studies involving vulnerable populations such as patients with Parkinson’s Disease (PD). This article delves into the recruitment process, ethical considerations, and detailed study design implemented at Juntendo University School of Medicine, where patients with advanced-stage PD were enrolled for an exploratory clinical trial.
Recruitment of Patients with Parkinson’s Disease
Between May and December 2021, a thoughtful recruitment process was executed to enroll patients from the Department of Neurology at Juntendo University School of Medicine. The focus was on patients with advanced-stage PD who exhibited fluctuating symptoms, primarily related to the "wearing-off phenomenon" of their primary medication, L-dopa.
Informed Consent
Central to the recruitment process was ensuring that all participants provided written informed consent. Each patient was thoroughly informed about the study’s purpose, procedures, and potential risks. This transparency was crucial not only for ethical compliance but also for fostering trust and collaboration between the researchers and participants.
Ethical Approval
The study received ethical approval from the Research Ethics Committee of the Faculty of Medicine at Juntendo University (H20-0376). Furthermore, it was registered with the University Hospital Medical Information Network-Clinical Trials Registry (UMIN000044246), ensuring that the trial adhered to regulatory standards and contributed to the field of clinical research.
Study Design Overview
This study utilized an uncontrolled, open-label, exploratory design. The two-phase approach included a baseline assessment of one week followed by a one-day blink evaluation. Patients underwent habituation with a specialized glasses-type device and were trained to maintain a symptom diary, fostering self-monitoring of their symptoms.
Treatment Protocol
On the blink evaluation day, each participant received a single dose of L-dopa/DCI in a fasting state, observing a 12-hour discontinuation of their regular anti-PD medication. The study aimed to assess blink information, fluctuations in motor symptoms related to PD, and the pharmacokinetics of L-dopa for a duration of four hours post-administration.
Inclusion and Exclusion Criteria
A total of 20 patients were recruited based on specific inclusion and exclusion criteria:
Inclusion Criteria
- Diagnosis: Patients had to meet the Movement Disorder Society (MDS) clinical diagnosis criteria for PD established in 2015.
- Stage of Disease: All participants were required to be at Stage ≤ III on the Hoehn and Yahr scale during the ON state.
- Treatment History: Participants had been treated with L-dopa for a minimum of six months and showed beneficial effects from this regimen.
- Inpatient Status: Eligible patients were those hospitalized for medical evaluation, medications adjustment, and rehabilitation.
- Consent Capability: Patients had to provide voluntary written informed consent based on sufficient understanding of the research.
- Visual Acuity: Participants had to be able to engage in evaluations without their personal eyeglasses, presuming they normally used glasses.
Exclusion Criteria
Participants were excluded if they had previous diagnoses or conditions such as atypical Parkinsonism syndromes, dementia, certain medical contraindications for L-dopa/DCI, psychiatric history, or previously received device-aided therapies. This careful selection ensured that the study population was as homogenous as possible.
Clinical Assessments
A comprehensive baseline assessment was performed on the subjects, gathering crucial data including:
- Personal Demographic Information: Age, gender, and body mass index.
- Disease Information: Duration of PD, MMSE scores, and levodopa equivalent daily doses.
- Stage of Disease: Rating based on the Hoehn-Yahr scale during both ON and OFF episodes.
Instruments Used
Multiple instruments were employed to assess patient symptoms, including:
- EuroQol 5 Dimensions 5-Level (EQ-5D-5L)
- Parkinson’s Disease Questionnaire-39 (PDQ-39)
- Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS)
- Unified Dyskinesia Rating Scale (UDysRS)
Additionally, patients were instructed to document their motor symptoms in a diary, according to a four-point scale, fostering an understanding of daily symptom fluctuations.
Pharmacokinetics Assessments of L-dopa
Prior to the blink evaluation, an overnight fasting period and a 12-hour medication-free interval were mandated. Subsequently, participants were administered a single dose of L-dopa/DCI consistent with their usual regimen. Blood samples were meticulously collected to analyze the pharmacokinetics of L-dopa at various time intervals.
Measurement Techniques
The plasma concentration of L-dopa was determined using high-performance liquid chromatography—a method that ensures accurate and reliable results vital for assessing therapeutic efficacy. The maximum concentration achieved (Cmax), time to reach maximum concentration (Tmax), and the area under the concentration-time curve (AUC0-t) were pivotal pharmacokinetic parameters extracted during patient evaluations.
Acquisition and Analysis of Pupil Data
Pupil data were collected using a state-of-the-art wearable eye-tracking headset, allowing for non-invasive monitoring of blinking patterns in relation to drug administration. The lightweight design ensured comfort during the four-hour monitoring period, providing valuable insights into the blink dynamics of PD patients.
Data Processing and Analysis
The recordings from the eye-tracking headset were processed using specialized software to extract precise blink data. This included the detection of blink onset and offset based on pupil visibility, ensuring robust data quality before analysis.
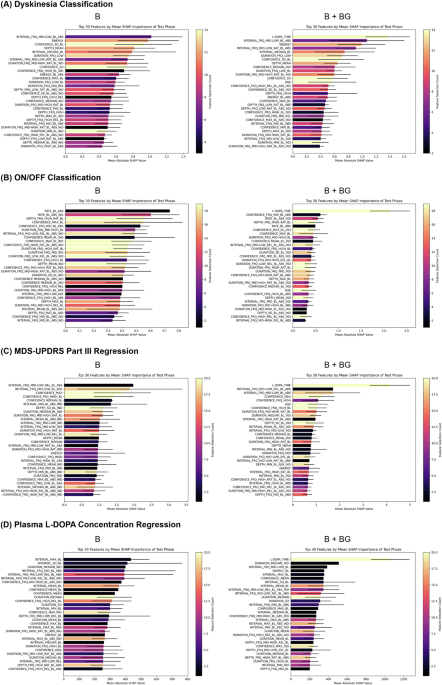
Machine Learning and Statistical Analysis
The study employed machine learning techniques to predict clinical outcomes based on blink features derived from the eye-tracking data. Various statistical methodologies were implemented to assess the predictive performance of multiple models, including classification and regression analyses.
Feature Extraction
A total of 468 blink-related features were extracted, comprising multiple statistical parameters related to blinks (e.g., blink rate, confidence levels) and additional non-blink variables such as plasma L-dopa concentration and patient age. Rigorous normalization methods were applied to prevent biases in the data analysis.
Conclusion
This structured overview of subject recruitment, ethical considerations, clinical assessments, and innovative methodologies demonstrates the careful planning and execution necessary for a successful clinical trial focused on Parkinson’s Disease. The multi-faceted approach not only aims to enhance understanding of the disease but also strives to improve patients’ quality of life through innovative treatment modalities.