Harnessing Machine Learning to Identify High-Risk HTLV-1 Carriers
Human T-cell Lymphotropic virus type 1 (HTLV-1) is a retrovirus associated with various diseases, including adult T-cell leukemia (ATL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM). Particularly concerning is the subset of asymptomatic HTLV-1 carriers who may have an elevated risk of progressing to HAM. In this article, we delve into an innovative machine learning-based approach designed to identify these high-risk carriers using anomaly detection algorithms and advanced statistical analysis.
Understanding HTLV-1 and Its Implications
HTLV-1 is primarily transmitted through infected bodily fluids, particularly via sexual contact, breast feeding, and blood transfusions. While most carriers remain asymptomatic, a notable portion will develop severe complications, like HAM and ATL, later in life. Monitoring asymptomatic carriers is critical, as identifying those at an increased risk for progression can significantly influence treatment and management strategies.
The Innovation of Anomaly Detection
In our study, we leveraged the Isolation Forest anomaly detection algorithm. This methodology focuses on identifying outlier data points—samples that are significantly different from others within the same category. By applying this algorithm to a dataset comprising asymptomatic HTLV-1 carriers, we successfully highlighted a subgroup of anomaly samples that exhibited characteristics similar to patients diagnosed with HAM.
This subgroup, dubbed "anomaly carriers," displayed antibody response patterns akin to those observed in HAM patients. This similarity raises critical questions about the trajectory of disease progression within these carriers.
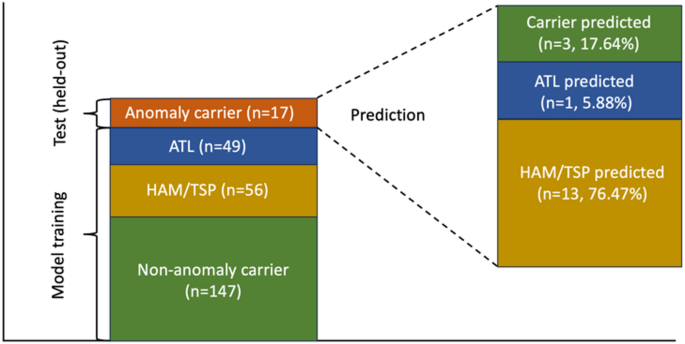
Characterizing the Anomaly Carrier Subgroup
The anomaly carriers were subjected to further characterization through classifier prediction and statistical analysis. The evidence strongly suggested that these carriers may be on a pathway to HAM progression. Notably, the classification accuracy was reinforced by the fact that many anomaly carrier samples were predicted as HAM by the Random Forest (RF) classifier. The inclusion of a purposely selected CDH sample—later diagnosed with HAM—further validated the hypothesis, as it was similarly identified as an anomaly.
Elevated Antibody Responses: An Indicator of Disease Trajectory
Antibody responses displayed notable differences between the asymptomatic carriers, anomaly carriers, and other clinical subgroups, allowing us to deepen our investigation into underlying risk factors. An interesting finding was that the anti-Env antibody titer levels in anomaly carriers significantly differed from those in HAM patients. The Env protein, vital for the virus’s transmission, is a primary target for the immune response, indicating that elevated levels of specific antibodies might be reflective of the immune system’s increasing activity as disease progression approaches.
Our results echoed previous studies that found elevated anti-Env antibody responses in HAM patients, forwarding the idea that these rising antibody levels may serve as a marker for individuals on the brink of developing symptomatic disease.
The Role of Shapley Additive Explanations (SHAP)
For comparative feature analysis among different sample groups (non-anomaly carriers, anomaly carriers, ATL, and HAM), we implemented SHAP. This powerful framework interprets model predictions and identifies the key driving features that differentiate each subgroup. In essence, it helps us understand which factors contribute most significantly to disease progression.
Noteworthy Results from SHAP Analysis
Our analysis revealed that the Tax protein emerged as a predominant feature among HAM patients—a result consistent with multiple studies that demonstrated its significance in disease pathogenesis. Additionally, proteins like Gag and Env were found to have inverse relationships with their anomaly scores, with higher feature values correlating with higher anomaly levels. This suggests that antibody responses to Gag proteins might serve as potential biomarkers for spotting high-risk carriers.
Heterogeneity Among Asymptomatic Carriers
One of the more profound revelations from our study was the significant heterogeneity in immune responses among asymptomatic HTLV-1 carriers. Surprisingly, we observed that many carriers exhibited antibody responses against HTLV-1 antigens comparable to those seen in individuals diagnosed with ATL and HAM. This extensive variability raises critical issues regarding the nature of immune responses and the lifestyle factors influencing them.
While the heterogeneity appears pronounced, our large dataset allows for a more nuanced understanding of these variations—highlighting the latent potential of asymptomatic carriers and their risk profiles for disease progression.
Limitations and Future Directions
As with any study, we faced limitations. We lacked long-term data on the anomaly carriers—besides one identified case who later developed HAM—which limits our ability to definitively link anomaly status to disease onset. Further prospective studies will be paramount to bolster our understanding. Moreover, although our method successfully isolated high-risk samples, the inherent complexity of immune responses and their interplay with HTLV-1 underscores the need for deeper exploration.
In summary, our study marks a vital step toward identifying asymptomatic HTLV-1 carriers at risk for HAM progression. By merging machine learning techniques with robust clinical analysis, we are paving the way for targeted interventions that could significantly enhance patient outcomes in the face of a complex viral landscape.