Neural Progenitor Cells in the Adult Human Hippocampus: Unraveling the Mystery of Neurogenesis
Neural progenitor cells have piqued the interest of neuroscientists for decades, primarily due to their capacity to develop into neurons. A recent transcriptomics study published in Science provides fresh insights into the presence of these cells in the adult human hippocampus, suggesting they persist into old age. This revelation adds weight to the idea that adults can, indeed, produce new neurons. However, skepticism remains about the significance of these findings, particularly regarding the rarity of these progenitor cells.
The Debate Over Neural Progenitors
Juan Arellano, a research scientist at Yale who did not participate in the study, acknowledges that there might be something noteworthy about the findings. However, he expresses concern about the practicality of these progenitor cells, stating that the research team relied on machine-learning algorithms to identify them. “Are they really so relevant in the circuit?” he questions, pointing to the rarity of these cells.
Despite the lack of quantification in the study, Ionut Dumitru from Karolinska Institutet argues that even a small number of newborn neurons could functionally contribute to the hippocampus. This hypothesis rests on the understanding that these neurons are highly excitatory and plastic, capable of influencing brain circuits.
Historical Context and Ongoing Research
The concept of adult neurogenesis isn’t entirely new. The first documentation of proliferating neurons in adults dates back to a 1998 study employing a synthetic nucleoside to track newly synthesized DNA in newborn cells. Several subsequent techniques, such as carbon dating and lineage tracing, have substantiated that human beings can continue generating new neurons after childhood. Yet, various studies utilizing cellular markers have yielded mixed results, leading many researchers to question the extent and role of neurogenesis in adult brains.
For instance, Shawn Sorrells from the University of Pittsburgh points out that many studies indicate a significant decline in neurogenesis shortly after birth, with relatively few neurons birthed in adulthood. This inconsistency has fueled ongoing debates in the scientific community.
Innovative Methodologies: The Role of Transcriptomics
Critics of earlier studies have argued that techniques focused on cellular markers may not be sensitive enough to detect progenitor cells in humans. A 2023 study suggests that transcriptomics—a method quantifying RNA transcripts—offers a more impartial way to capture rare cell populations. This method allows researchers to bypass the limitations of previous markers and gain a deeper understanding of complex cell types.
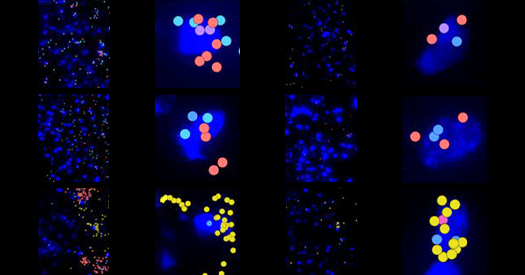
In this recent study, researchers took an innovative approach by isolating proliferating cells from human brains, thereby optimizing their chances of identifying progenitors. By employing single-nuclei RNA sequencing, they profiled neural progenitor cells from the hippocampi of children aged five and younger. Their findings were then cross-referenced against sequencing data from adults aged 20 to 78, which were analyzed using machine-learning algorithms.
Mapping the Complexity of Neural Progenitors
Evgenia Salta, a group leader at the Netherlands Institute for Neuroscience, noted that the latest findings provide the most comprehensive molecular profile of these cells to date. The researchers found several types of progenitor cells—neural stem cells, immature progenitor cells, and neuroblasts—each with a unique expression profile and spatial distribution.
Remarkably, the presence of these progenitor cells varied significantly among individuals, with five participants exhibiting no detectable cells at all. This variability raises questions about the biological consistency of neurogenesis across different adults.
The Complexity of Identifying Progenitor Cells
Despite the promising methodologies, the identification of progenitor cells remains challenging. For instance, the expression of Doublecortin, frequently used as a marker for immature neurons, dilutes the specificity necessary to pinpoint these cells. Marta Paterlini, also from Karolinska Institutet, noted that a multifaceted approach—utilizing a variety of markers—is essential for accurately defining progenitor cells.
Salta emphasizes the necessity of acknowledging the complexity of the human brain. “We will never be able to find this one cell-type-specific marker for the human neurogenic populations,” she explains. The overlap of progenitor cell expression profiles with other cell types, like astrocytes and immune cells, adds another layer of complexity to the ongoing debate.
Individual Variability and Future Directions
While some experts remain skeptical of the robustness of adult neurogenesis, particularly given the necessity of advanced techniques to identify progenitor cells, others see a silver lining. Salta suggests that the observed variability across individuals might reflect underlying biological diversity, warranting further exploration. For instance, the intriguing connection between higher levels of immature progenitor cells and conditions like epilepsy prompts valuable questions about the impact of neurological conditions on neurogenesis.
In summary, while the debate about the significance of neural progenitor cells in adult brains continues, recent findings offer a compelling glimpse into the complexities of neurogenesis. As researchers employ more sophisticated methodologies, we move closer to understanding not only the mechanisms behind neurogenesis but also its potential implications for brain health across the lifespan.