Challenges in Managing Ground-Glass Nodules: Embracing Innovation Through Technology
Ground-glass nodules (GGNs) present a significant diagnostic challenge in clinical practice, primarily due to the complexities of overdiagnosis and the complications that arise from excessive follow-up procedures. Often, these management practices result in unnecessary interventions that place a considerable psychological and emotional burden on patients. As medical professionals grapple with these challenges, recent studies, including innovative models like radiomics and deep learning techniques, are showing promise in the quest to improve patient outcomes.
Understanding Ground-Glass Nodules (GGNs)
Most GGNs exhibit stability over extended periods. However, there are instances where invasive adenocarcinomas (IACs)—which may involve vascular or stromal invasion—emerge from these nodules. Detecting such transformations is crucial, as they can even result in lymph node metastasis. The imperative lies in the early and accurate identification of IACs to optimize treatment decisions and enhance prognoses for patients. Currently, conventional computed tomography (CT) imaging features such as size, density, and morphology are primarily relied upon for assessing invasiveness. Yet, these methods are inherently subjective and can differ based on a radiologist’s expertise, leading to interobserver variability.
The Evolution of Predictive Models
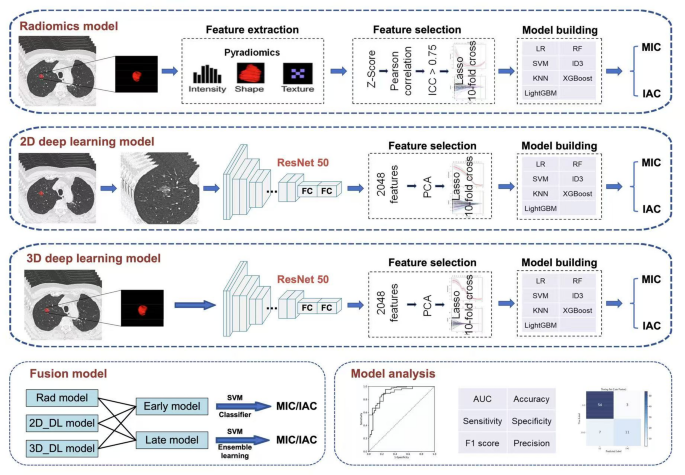
Traditional methods of predicting the invasiveness of GGNs often yield areas under the curve (AUCs) averaging around 0.7, highlighting their limitations. By contrast, recent advances in radiomics and deep learning methodologies have shown improved performance, thanks in part to their ability to extract high-dimensional features that surpass human perception.
In a recent study, researchers evaluated various predictive models including radiomics, 2D deep learning, and 3D deep learning, ultimately concluding that the late fusion model, which combines these techniques, achieved an impressive AUC of 0.898. This late fusion approach integrates features from both radiomics and deep learning, paving the way for more accurate identification of IACs, particularly in GGNs containing less than 50% solid components.
The Role of Artificial Intelligence
As the demand for advanced diagnostic methods grows, artificial intelligence (AI) is becoming increasingly relevant in the realm of lung cancer. Not only does it assist in identifying tumors more efficiently, but it also offers insights into tumor heterogeneity and risk stratification, making it a powerful ally in oncology.
Our study demonstrated that the 3D deep learning-based support vector machine (SVM) model exhibited superior performance in classifying GGNs as either minimally invasive adenocarcinomas (MIAs) or more aggressive IACs. This development resonates with findings from other studies revealing that models leveraging 3D deep learning consistently outperform alternative methods due to their capacity to capture volumetric spatial context and enhance the characterization of tumor heterogeneity.
Advantages of Late Fusion Models
One of the significant advancements in this area is the application of late fusion strategies, which have been shown to outperform early fusion strategies in predictive accuracy, especially when analyzing diverse imaging datasets. Late fusion allows for the independent processing of data modalities, ensuring that one does not disproportionately influence the others. This adaptability proves especially beneficial in situations where data may be incomplete or heterogeneous.
Recent analyses demonstrate that 63% of evaluated late fusion methods performed favorably compared to single-modality models, reinforcing the efficacy of combining various datasets to improve diagnostic precision.
Broadening Horizons Beyond Lung Cancer
While the focus of this study is on lung adenocarcinomas, it’s noteworthy that similar techniques in radiomics and deep learning are gaining traction across multiple oncology disciplines. These methodologies are employed not just in lung cancer, but also in breast cancer, prostate cancer, and even gastrointestinal malignancies, significantly broadening the horizons for predictive modeling in oncology.
For instance, preoperative CT images have been utilized in a radiomics model to classify rectal cancer risks, while 3D deep learning applications are emerging in areas like prostate and brain tumor assessments. These cross-disciplinary applications underscore the versatility and potential impact of these advanced analytical methods on cancer diagnostics.
Limitations of Current Research
Despite the promising advances in AI and imaging, the findings of the study bear limitations. The retrospective nature of the investigation, conducted within a single-center environment, raises concerns about the generalizability of the results across different clinical settings. Additionally, the focus on surgically resected cases may introduce selection bias, as it limits applicability to patients who might be managed conservatively or those undergoing non-surgical approaches.
The study’s exclusion of other adenocarcinoma types or early pre-invasive lesions presents another challenge, potentially narrowing the model’s scope of applicability. Furthermore, concerns regarding model overfitting and variability stemming from manual segmentation processes must be addressed to enhance robustness.
Future Directions in GGN Management
This ongoing exploration into the use of combined radiomics and deep learning models represents a significant shift in how GGNs may be managed in the future. By moving towards non-invasive and reproducible approaches, such models are poised to assist healthcare practitioners in conducting preoperative risk stratification, ultimately supporting tailored treatment strategies.
As exploration continues, it remains crucial to validate these models through larger, multi-institutional studies, ensuring that innovative approaches can address the pressing challenges currently faced in the clinical management of GGNs. The integration of advanced technologies like AI into standard practices holds the potential not just for improved diagnostics, but for a more personalized approach to cancer care overall.