Design of Combinatorial Variants of PylRS Using the FFT-PLSR Model
Introduction to PylRS and Importance of Mutations
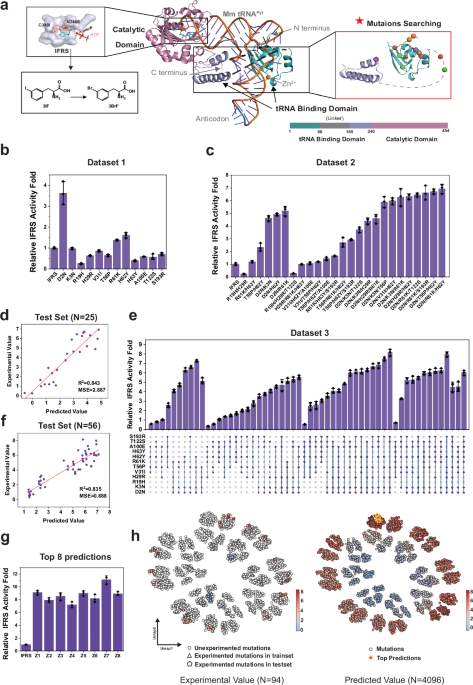
Pyrrolysyl-tRNA synthetase (PylRS) plays a crucial role in incorporating non-canonical amino acids (ncAAs) into proteins, providing opportunities for novel biochemical functions. In search of enhancing the efficiency of this incorporation process, researchers have identified mutations in the tRNA-binding domain (TBD) of Methanosarcina acetivorans (Mm) PylRS that boost catalytic efficiency. These mutations, especially those located in the N-terminal region, surprisingly do not detract from substrate specificity and are transferrable across different variants.
The Mutation Framework
The focus turned to four specific sets of mutations previously identified for their capacity to enhance the efficiency of translational frameshift and stop codon suppression (SCS): R61K/H63Y/S193R, R19H/H29R/T122S, D2N/K3N/T56P/H62Y, and V31I/T56P/H62Y/A100E. These mutations were incorporated into an improved form of the coding enzyme, referred to as in-frame ribosome synthetase (IFRS). Each variant underwent rigorous testing with 3-bromo-phenylalanine (3BrF), a cost-effective substrate, enabling a tighter assessment of their functional efficacy.
Expression Systems and Assay Methodology
For the experiments, an expression system was established using a constitutive mid-strength E. coli promoter for the IFRS. The activity was evaluated via fluorescence intensity measurements of sfGFPS2TAG, a fluorescent reporter gene, and fluorescence intensity to optical density (OD) ratios were calculated to quantify the yield of ncAA-containing proteins. Notably, while some mutations like D2N/K3N/T56P/H62Y resulted in a marked increase in SCS activity, others did not yield the anticipated enhancements.
The proteins were categorized based on their efficiencies, discovering that D2N and H62Y exhibited potential benefits—but not all mutations positively impacted activity. Models demonstrated a complex interplay among the mutations, emphasizing epistasis effects that could either enhance or inhibit protein functionality.
Machine Learning Application: FFT-PLSR Model
Amidst these experiments, an innovative approach using the Fast Fourier Transform (FFT) integrated into a Partial Least Squares Regression (PLSR) model emerged. This model harnesses machine learning (ML) to predict activity across numerous combinatorial variants derived from 12 single-point mutations, theoretically producing 4,096 distinct enzyme constructs.
Subsequent training of the FFT-PLSR model with existing datasets revealed strong predictive capacity, leading researchers to construct double and triple mutants based on initial yield data. The model reached notable accuracy levels, foreshadowing an efficient strategy for discovering high-activity variants within the vast sequence space.
In dataset analysis, certain unknown epistatic relationships were uncovered, which proved invaluable as researchers began to merge datasets, enabling a richer understanding of how mutations interact within the protein structure.
Deep Learning Enhancements
With robust machine learning frameworks established, the next step was to employ deep learning models, specifically ESM-1v, MutCompute, and ProRefiner, for zero-shot predictions of high-fitness variants exerting influence beyond the training dataset’s scope. The diversity these models brought enhanced the potential for significant insights.
Researchers trained these models on single-point variants and constructed 95 mutants across pivotal regions of Com1-IFRS, capturing a wealth of activity data in the body of research. Enhancements tracked by the models opened doors to novel experimental constructs that could vastly improve enzymatic activity.
Interestingly, while many predicted variations showed initial promise, a fair number lacked functional contributions, revealing limitations in the predictive power tied to unseen mutation sites.
Exploring Molecular Changes Through MD Simulations
Turning to the molecular level, MD simulations were employed to visualize structural changes induced by the mutations. Utilizing AlphaFold3 for structural modeling provided valuable insights into changes occurring in the PylRS complex during tRNA interactions. Distinct mutations translated to observable differences in binding efficiencies and mutation-driven enhancements, as evidenced by the emergence of new hydrogen bonds.
Simulation results indicated that mutated variants like Com1 and Com2 showed decreased distances that favorably align with enzymatic substrates, showcasing their improved catalytic profiles. Tracking hydrogen bond formation and stability through these simulations articulated the nuanced dynamics at play within the enzyme’s structure.
Suppression of Amber Codons
The next exploration revolved around the suppression capabilities of Com1-IFRS and Com2-IFRS concerning multiple amber codons. This suppression is critical for the incorporation of multiple ncAAs, offering a unique chance to push boundaries in bioengineering. The experimental evaluations were enlightening, revealing improved efficiencies in scenarios with increased consecutive amber codons.
These findings underscore the versatile capabilities of engineered PylRS variants, as both Com1 and Com2 enable the incorporation of diverse ncAAs into proteins, reinforcing their potential application as tools in synthetic biology.
By embracing the cutting-edge fusion of mutation-driven enzymatic design and advanced computational predictive modeling, researchers are incrementally paving a path toward the more effective synthesis of proteins that leverage the vast array of ncCAs. This study stands as a testament to the profound potential harbored within the junctions of machine learning, synthetic biology, and protein engineering. As these methodologies continue to evolve, they promise to transcend traditional constraints, enabling the design of proteins capable of novel functions and applications in a variety of scientific fields.