Enhanced Prognostic Capability: Deep Learning Models in the Evaluation of Ground-Glass Nodules (GGNs)
Recent advancements in technology are transforming how healthcare professionals assess and predict the behavior of ground-glass nodules (GGNs) in computed tomography (CT) images. A groundbreaking study published in the European Journal of Radiology showcases a multiple time-series deep learning (DL) model that significantly outperforms traditional clinical and radiomic models in prognostic capabilities regarding GGNs.
The Study Overview
The retrospective study involved a comprehensive review of 1,031 CT scans from 486 patients, with an average age of 56.7 years. Researchers compared various models, including the deep learning model, a clinical model, a delta radiomic model, and a combined model. The aim was to analyze how effectively these models could differentiate between malignant and benign GGNs.
Key Findings on Sensitivity
In terms of sensitivity for predicting GGNs, the results were telling. The deep learning model achieved an impressive sensitivity rate of 88%, compared to 84% for the combined model. In contrast, the delta radiomic model and the clinical model lagged behind at 68% and 32%, respectively. This indicates that the DL model is exceptionally effective in identifying malignant nodules early, which is crucial for timely interventions.
Specificity and Positive Predictive Value (PPV)
While sensitivity measures the model’s ability to correctly identify positives, specificity and positive predictive value (PPV) are equally important metrics. In this study, the clinical model excelled with a specificity of 95.8% and a PPV of 88.9%. However, the DL models demonstrated lower specificity and PPV, coming in at 75% and 78.6%, respectively. This highlights a trade-off between the ability to identify positives and the accuracy of those predictions.
Negative Predictive Value (NPV) and Area Under the Curve (AUC)
One of the notable advantages of the DL model is its negative predictive value (NPV). The model exhibited a remarkable 85.7% NPV, significantly outperforming the clinical model’s 57.5%. Additionally, the DL model’s Area Under the Curve (AUC) was recorded at 81.7%, compared to 61.2% for the clinical model. These findings reinforce the model’s ability to rule out malignancy effectively, thus minimizing unnecessary follow-ups and interventions.
Temporal and Semantic Features Integration
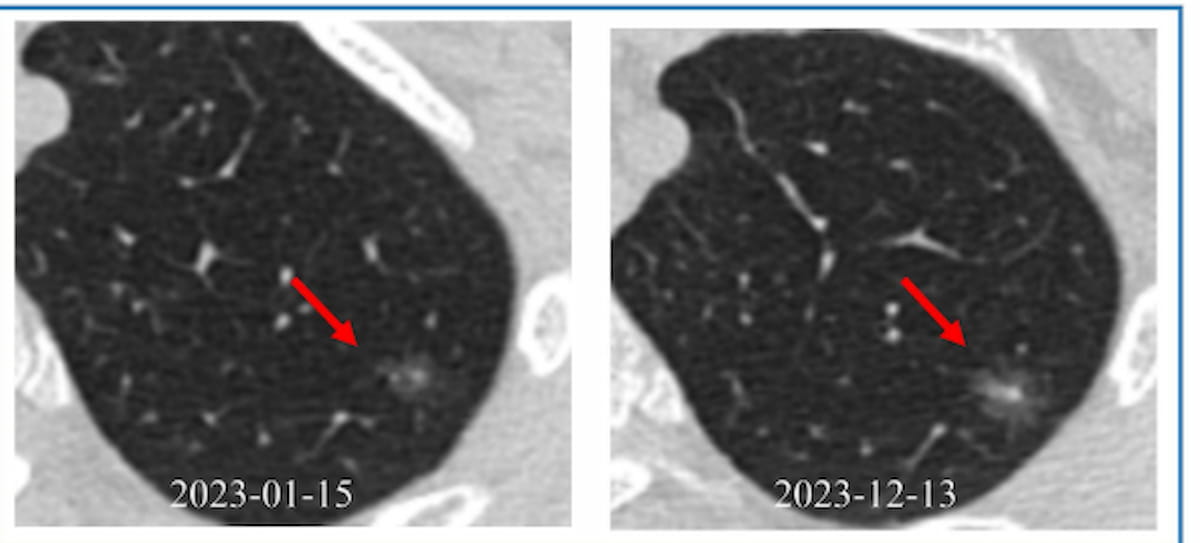
Dr. Xiaolong Yang, the lead author of the study, emphasized that the DL model excels because it analyzes CT images over multiple time points. This temporal dimension allows for a comprehensive assessment of longitudinal changes in lesions. By integrating clinical information with image-derived semantic features—such as lobulation and density heterogeneity—the model becomes a powerful tool in predicting malignancy.
Distinguishing Malignant from Benign GGNs
The researchers also uncovered specific characteristics that distinguish malignant from benign GGNs. Malignant GGNs showed a higher prevalence of part-solid nodules (59.8% vs. 36.6%) and heterogeneous density (71.3% vs. 47%). Lobulation, a significant feature, was also more common in malignant nodules (81.9% vs. 31%). These features enhance the model’s predictive accuracy, allowing clinicians to better differentiate between aggressive and indolent nodules.
Potential Implications for Clinical Practice
The implications of these findings are far-reaching. By minimizing false positives and unnecessary interventions, the DL model supports a more personalized "watch-and-wait" strategy. This optimizes resource utilization while ensuring that patients receive the most appropriate level of care.
Limitations of the Study
Despite promising results, the authors acknowledge limitations inherent to the study’s retrospective nature. The cohort solely consisted of patients who had undergone lung nodule resection, which may introduce selection bias.
As healthcare continues to evolve with technological advancements, studies like this demonstrate the potential of deep learning to enhance diagnostic accuracy in critical areas such as oncology. The enhancement of prognostic capabilities using multiple time-series analyses not only holds promise for better patient outcomes but also encourages a shift toward more personalized and efficient healthcare practices.