“Enhancing Intraoral Free Flap Monitoring with Machine Learning Techniques for Imbalanced Clinical Data”
Enhancing Intraoral Free Flap Monitoring with Machine Learning Techniques for Imbalanced Clinical Data
Understanding Intraoral Free Flap Reconstruction
Intraoral free flap reconstruction involves transplanting tissue from one part of the body to another to repair defects in the oral cavity, often post-surgery for conditions like cancer. This technique is vital for both functional recovery, such as restoring speech and swallowing, and aesthetic purposes. Despite its success rate exceeding 95% (Lutz & Wei, 2005), complications such as vascular compromise can lead to significant tissue loss and aesthetic issues.
The Challenge of Monitoring
Monitoring the condition of free flaps is crucial to detect complications early. Traditional monitoring methods include visual inspection, Doppler ultrasound, and near-infrared spectroscopy. However, each has its limitations: they can be difficult to interpret, require equipment that may not be portable, or fail to provide quantifiable data on flap viability (Chae et al., 2015). The lack of effective monitoring techniques can lead to missed diagnoses, ultimately resulting in flap loss and the need for additional surgeries.
Machine Learning Approach to Imbalanced Data
Recent advances in machine learning provide powerful tools for enhancing flap monitoring. One of the critical challenges in this domain is the imbalance in clinical data, where cases of flap failure are rare compared to successful outcomes. In response, machine learning models can be trained to recognize patterns indicative of complications even when these events are infrequent.
For instance, a model developed by Hsu et al. utilized a supervised learning framework to quantify changes in extraoral flap viability. Although previously untested for intraoral flaps, this approach addresses class imbalance by using techniques like class weighting and focal loss during training. Class weighting assigns more importance to less frequent cases, while focal loss helps focus learning on challenging instances, improving classification accuracy.
Step-by-Step Process of Implementation
-
Data Collection: Data must be collected from clinical images of intraoral free flaps. These images should capture various states of the flap under different conditions (e.g., lighting, presence of blood).
-
Preprocessing: The images should be preprocessed to standardize conditions, such as adjusting for lighting variations. Random adjustments can be implemented to ensure robust model training.
-
Model Training: Using a convolutional neural network (CNN), the model learns to identify changes in flap conditions based on the training data. Incorporating techniques to manage class imbalance ensures that the model adequately recognizes cases of vascular compromise.
-
Testing and Validation: Rigorous testing using a separate set of images is essential to evaluate the model’s performance, specifically its ability to distinguish between healthy flaps and those at risk.
- Clinical Deployment: Finally, the model must be calibrated for clinical use, ensuring it provides reliable guidance for surgical teams in real-time decision-making.
Case Examples in Practice
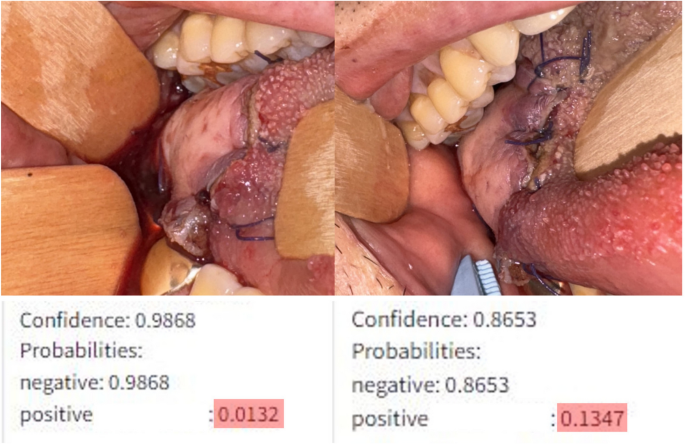
In a clinical study involving 131 patients, the machine learning model showed promising results. It recognized subtle differences in flap vitality that human observers missed. For example, even slight changes in color or edge definition were detected, prompting timely interventions, which prevented complications in several cases. This real-time assessment not only enhanced patient safety but also reduced unnecessary surgeries.
Common Pitfalls and Recommendations
One significant challenge is the model’s recall rate for identifying vascular compromise, which was observed to be 0.83, suggesting a 17% false-negative rate (where actual compromises are misclassified as healthy). This misclassification stems partly from a limited dataset, which lacks varied examples of complications. Adjusting the confidence thresholds for what the model flags as compromised can help mitigate this issue, accepting a higher false-positive rate to prioritize patient safety.
Technical Tools and Frameworks Used
The tools used for creating these machine learning models typically include TensorFlow and PyTorch, popular frameworks in deep learning. These platforms offer robust resources for handling image data, optimizing neural network structures, and managing computational demands. By employing these technologies, healthcare professionals can harness the power of AI to enhance clinical outcomes.
Alternative Approaches and Their Trade-offs
While machine learning offers significant advancements, alternative monitoring technologies, like traditional Doppler systems or emerging infrared spectroscopy tools, can still serve complementary roles. However, each comes with trade-offs regarding portability, cost, and ease of use. The choice between these methods largely depends on the clinical context, resources available, and specific patient needs. Machine learning models shine in their adaptability and sensitivity to rare events, making them a key asset in enhancing flap monitoring.
Frequently Asked Questions
What are the benefits of using machine learning for intraoral flap monitoring?
Machine learning provides enhanced precision in detecting complications, especially those that are difficult to ascertain through traditional methods. This can lead to earlier interventions and better patient outcomes.
How do you manage class imbalance in training data?
Class imbalance can be addressed through techniques like class weighting and focal loss, which increase the model’s sensitivity to less frequent cases and improve overall classification rates.
Is this technology already in clinical use?
While promising results have been reported in studies, clinical deployment requires further validation and calibration based on specific patient demographics and clinical settings.
What limitations exist for machine learning in this context?
Limitations include potential misclassifications and the need for diverse training data to generalize across different patient profiles accurately. Continuous evaluation and adjustment of the model’s parameters are necessary for optimal performance.