Overview of River: A Cutting-Edge Tool for Spatial Omics Analysis
Introduction
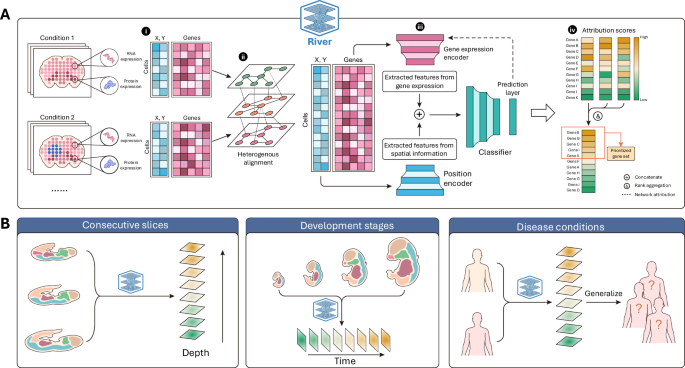
In the rapidly advancing field of spatial omics, River emerges as a powerful tool for analyzing complex biological data. By leveraging spatial omics data, the system interprets gene expression information based on specific spatial locations of cells, facilitating deeper insights into biological processes.
Functional Modules of River
River consists of two main functional modules that work in tandem to facilitate sophisticated analysis:
-
Prediction Model:
This module employs a Multi-Layer Perceptron (MLP) to accurately map spatial omics features (like transcriptomics and proteomics) along with their spatial coordinates to condition labels. The training process demands spatial omics data, which includes detailed feature values, spatial coordinates for each single cell, and condition labels (e.g., phenotypes). It’s noteworthy that River is ideally suited for comparative studies, requiring diverse biological conditions rather than being limited to single conditions or technical replicates. - Attribution Methods:
Once trained, the model employs various attribution methodologies to elucidate which genes are pivotal in determining the predictions for each input cell. By computing cell-wise gene scores, River analyzes the relevance of each gene across inputs, ultimately producing a ranked list of genes. This process involves rank aggregation methods to establish a comprehensive ranking across the different attribution techniques.
Handling Multiple Input Slices
River efficiently manages varying spatial coordinate systems among multiple input slices. To align these slices for analysis, it utilizes the Spatial-Linked Alignment Tool (SLAT), ensuring flexibility in aligning heterogeneous slices. This process includes designating a primary (base) slice and aligning other slices relative to that standard. SLAT generates matching lists that project the coordinates of the remaining slices into the same spatial coordinate system as the base slice.
Data Preprocessing
For optimal functionality, spatial omics data needs proper preprocessing. For example, raw-count spatial transcriptomics data requires normalized input to enhance stability during training. Normalization methods like scanpy.pp.normalize_total and scanpy.pp.log1p are recommended. On the other hand, spatial proteomics data typically comes pre-normalized, though L2 normalization is advised for raw datasets. By addressing batch effects and normalizing gene scores, River ensures the consistency and reliability of outputs.
Prediction Model Architecture
The architecture of River’s prediction model is composed of gene expression vectors, corresponding labels, and aligned coordinates. The system uses separate encoders for gene expression and positional information, combining these inputs to derive a latent expression that captures spatial-aware features. Each latent vector contributes to the final predictions, which are computed using a cross-entropy objective during training.
Attribution Techniques
River employs multiple attribution methods to identify Differential Spatial Expression Pattern (DSEP) genes. These methods hinge on the assumption that only genes with pronounced spatial expression shifts can significantly influence classification outcomes. The approach includes gradient-based techniques that provide efficient, robust measures of gene importance, thereby enhancing the reliability and comprehension of the model’s predictions.
Methods Utilized
- Integrated Gradients
- DeepLIFT
- GradientShap
These methods yield weight vectors that signify the importance of each gene for the model’s outcomes. Cell-level attribution scores are normalized for consistency, and global attribution scores are derived by averaging across multiple cellular evaluations.
Rank Aggregation
To merge results from various attribution methods, River employs the Borda count method, which quantifies each gene’s importance by aggregating scores from different rankings. This method facilitates the synthesis of diverse perspectives into a cohesive view on gene importance, yielding a robust final ranking delineating each gene’s contribution.
Outcome Gene Set Selection
River does not generate significance p-values but provides ranked gene listings. Users can either manually select top-k ranks or employ the Elbow point method for automatically determining cutoffs based on the score curve’s characteristics.
Simulation Dataset
In practical applications, River employs simulated datasets to benchmark performance. For instance, control slices featuring distinct spatial domains are generated, after which differential expression patterns are induced in datasets. This simulation facilitates robust testing of River’s efficacy in detecting meaningful spatial expression variations.
Implementation Details
When working with benchmark datasets, River seamlessly integrates slices without requiring pre-alignment, whereas real data experiments use SLAT for proper coordination. The system incorporates dropout regularization and an efficient training regimen to foster optimal model performance.
Comparative Analysis
In demonstrating River’s efficacy, it’s aligned against leading methodologies across three categories: High-Variable Genes (HVG) detection, Spatially Variable Genes (SVG) identification, and three-dimensional spatial analysis techniques. Comparative results underscore River’s proficiency in recognizing differential spatial expression patterns against well-established baseline methods.
Evaluation Metrics
To assess River’s performance, the F1-score is employed as the primary metric when identifying DSEP genes. This scoring method simplifies the evaluation process, offering a comprehensive analysis of model accuracy across various experimental conditions.
Disease and Developmental Applications
River’s versatility is showcased through its application to datasets linked to developmental biology and disease contexts, such as mouse embryo stages and diabetes-induced alterations in testis. Here, gene set enrichment analyses further illuminate the biological implications of River’s findings, demonstrating its applicability in clinical settings.
Conclusion
River stands as a significant advancement in spatial omics analytics, effectively marrying machine learning principles with biological inquiry. By enabling precise predictions and actionable insights into gene behaviors across diverse spatial contexts, River empowers researchers to explore the complexities of biological systems in unprecedented detail.