Early Detection of Alzheimer’s Disease Using Deep Learning and MRI
Introduction
Alzheimer’s disease (AD), a debilitating neurodegenerative condition, presents significant challenges for early diagnosis and treatment. Recent advancements in neuroimaging and deep learning technologies offer promising avenues for early detection. This article explores the innovative use of structural MRI data alongside advanced machine learning models for diagnosing Alzheimer’s disease.
Methodology Overview
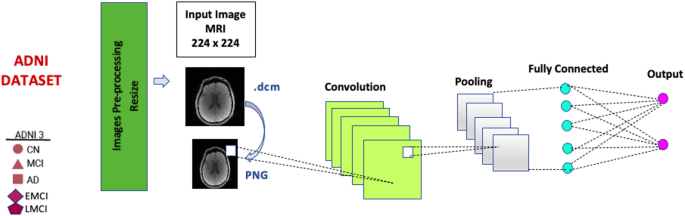
In this study, a variety of convolutional neural network (CNN) architectures, including EfficientNetB2, InceptionV3, and RegNetX006, were utilized to analyze 24,661 MRI scans obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI-3) database. Each MRI image was rescaled to dimensions of 224 × 224 pixels to meet the input requirements of these models.
Data Preprocessing
The initial step involved transforming DICOM images with the .dcm extension into PNG format to facilitate processing. The preprocessing phase was crucial for ensuring that the data fed into the models was standardized, enhancing the reliability of the outcomes. Moreover, a 10-fold cross-validation technique was employed to effectively partition the dataset into diverse subsets, allowing for robust training and testing cycles.
-
K-Fold Cross-Validation: Partitioning the dataset into 10 subsets (or folds), each used as a test set while the remaining folds are employed for training, ensures that the model is validated rigorously. The average accuracy across these folds provides a comprehensive measure of model performance.
- Stratified Sampling: To address potential class imbalance, stratified sampling ensured that each fold contained representations from all classes, thus maintaining the ratio of different stages of Alzheimer’s disease in the sample set.
Model Training
The training of the models was conducted on Google Colab, utilizing the TensorFlow and Scikit-learn libraries. An NVIDIA A100 GPU with 80 GB of High Bandwidth Memory significantly enhanced computational efficiency, allowing complex neural networks to process large medical datasets rapidly.
Dataset Insights
The ADNI-3 Dataset
The ADNI-3 dataset is a rich resource that includes comprehensive information on 627 participants categorized as follows:
- Alzheimer’s Disease (AD): 50 individuals
- Early Mild Cognitive Impairment (EMCI): 37 individuals
- Mild Cognitive Impairment (MCI): 160 individuals
- Late Mild Cognitive Impairment (LMCI): 15 individuals
- Cognitively Normal (CN): 365 individuals
This dataset presents an invaluable opportunity for training models, as each participant has an extensive collection of MRI slices—54 slices each, encompassing longitudinal imaging across multiple years.
Understanding Class Labels
- AD: Individuals diagnosed with Alzheimer’s disease.
- CN: Healthy controls without cognitive impairment.
- MCI and LMCI: Stages of cognitive impairment that serve as precursors to Alzheimer’s, where early diagnosis is especially crucial.
Structural Similarity Index Measure (SSIM)
To enhance the quality of MRI slice selection for model training, the Structural Similarity Index Measure (SSIM) was employed. SSIM provides a quantitative measure of similarity between two images based on luminance, contrast, and structure. This index aids in selecting the most informative slices for analysis, enhancing the model’s capability to detect subtle morphological changes characteristic of Alzheimer’s disease.
- Selection Process: Five slices were manually evaluated, focusing primarily on the middle slices of the 54-slice MRI stack. The slice with the highest count of edge segments, as determined by the Canny edge detector, was chosen to ensure robust anatomical representation.
Advanced Model Architectures
The study investigated several models, including a new model integrating Feature Pyramid Networks (FPN) into the EfficientNetB2 architecture. FPN facilitates the recognition of objects at various scales within the brain images by combining feature maps from different layers, thereby enhancing diagnostic performance.
Key Model Highlights
- EfficientNetB2: A powerful model known for its optimal parameter efficiency.
- InceptionV3 and RegNetX006: Established architectures that provide competitive baselines in classification tasks.
Proposed Enhancements
The authors introduced innovative modifications by combining EfficientNetB2 with an FPN architecture. The multi-scaling capabilities of FPN allow for capturing both large-scale and small-scale anatomical changes crucial for early AD detection. The architecture was configured to assimilate different resolutions of feature maps, effectively merging high- and low-level features.
Performance Metrics
To evaluate the models’ effectiveness, multiple performance metrics were employed:
- Accuracy: Represents the ratio of correctly classified instances to the total instances.
- Precision and Recall: Provide insight into the models’ capabilities in identifying positive cases.
- F1-score: Balances precision and recall, enhancing evaluation accuracy.
- ROC-AUC: Measures the area under the ROC curve, giving a graphical indication of the model’s performance across various thresholds.
These metrics guide researchers in comprehensively assessing models and refining techniques for greater accuracy.
Statistical Methods
McNemar’s Test
McNemar’s Test serves as a valuable tool to evaluate whether one model significantly outperforms another in binary classification tasks. By analyzing discordant pairs, it provides researchers with insights into performance differences, emphasizing statistical significance over chance.
Innovative Model Development
A secondary model combining Vision Transformer (ViT) architecture with EfficientNetB2 and FPN was crafted. Utilizing transformer encoders allowed for improved global context learning of spatial features, thus enhancing overall classification capabilities. The implementation of multi-head self-attention within the ViT framework allows for the aggregation of local features while maintaining spatial relationships, a crucial aspect of MRI analysis.
Conclusion
Through the integration of advanced deep learning architectures and comprehensive datasets, this study underscores the potential for early and effective diagnosis of Alzheimer’s disease via MRI analysis. By employing robust methodologies, including innovative slice selection techniques and sophisticated model constructions, researchers are making significant strides towards enhancing detection accuracy, ultimately offering hope in the fight against neurodegeneration.