Cell Culture and Gene-Editing Technology: A Modern Exploration
Cell Culture
Cell culture is an essential technique in biological research, enabling scientists to study cellular processes in controlled environments. One widely used cell line is HEK293T, derived from human embryonic kidney cells. As recommended by the American Type Culture Collection (ATCC), HEK293T cells (ATCC, CRL-11268) are cultured under specific conditions to ensure optimal growth and viability. Notably, these cell lines have been tested and confirmed free of Mycoplasma, ensuring the integrity of experimental data. Furthermore, they have been authenticated by their suppliers, which adds an extra layer of reliability to research findings.
Modeling Gene-Editing Outcomes
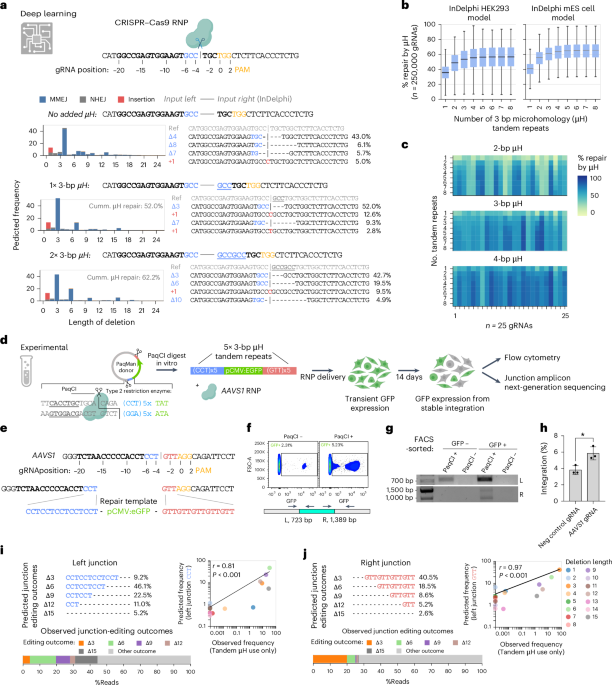
In the field of gene editing, modeling expected outcomes can significantly enhance experimental design. The inDelphi model, accessible on GitHub, serves as a predictive tool for studying various gene-editing scenarios. Deployed within a proper Python virtual environment, this model allows researchers to examine the effects of various tandem repeat lengths on DNA repair efficiencies.
To quantify the impact of tandem repeats on DNA repair outcomes, custom Python code was developed. This code defines the percentage of repair by µH (micro-homology) as the sum of repair outcomes utilizing at least one tandem repeat. By iterating through tandem repeat lengths and quantities, the code evaluates multiple scenarios using the first 250,000 gRNA sites identified by the presence of NGG PAM in human transcript sequences.
For HEK293T experiments, predictive modeling was conducted using the inDelphi model tailored for these cells. Conversely, for modeling in Xenopus and murine embryos, the predictive model used was validated for mouse embryonic stem cells (mESC), showcasing its adaptability in early-dividing organisms.
Cloning and In Vitro Linearization of Repair Plasmids
The process of gene editing often requires precise repair templates. In this research, a donor plasmid was assembled in a pUC19 backbone using Gibson cloning for optimal integration. This plasmid includes a pCMV-eGFP transgenic cassette, flanked by µH tandem repeat repair arms, facilitating targeted integration via CRISPR technology.
Linearization of the donor plasmid was performed with the PaqCI restriction enzyme, ensuring correct size and structure for downstream applications. Alternatively, repair templates containing µH tandem repeats were generated using overhang PCR.
For the in vivo applicability of these templates, PCR products required purification—different methods were utilized for both in vitro and in vivo preparations to ensure high-quality products for subsequent experiments.
µH Tandem Repeat-Mediated Integration In Vitro
Gene editing using CRISPR technology involves assembling the gRNA and Cas9 protein, and introducing repair templates into target cells. In the case of HEK293T cells, the RNP complex comprising the gRNA duplex, Cas9 protein, and repair template was prepared and introduced via reverse transfection.
By maintaining specific parameters and incubation times, researchers aimed to achieve successful integration of the transgene, monitoring outcomes through the expression of eGFP in sorted cells after a specified culture period.
In contrast, for TRAC CAR knock-in experiments, slight modifications were made to the assembly and transfection process, tailoring the protocols to fit the specific requirements of different target genes and plasmid constructs.
Gene Editing Targeting Strategies
To ensure efficient targeting for gene editing, researchers employed a custom Python script to design gRNAs for the human genome. Each gRNA was filtered based on specific criteria to maximize efficiency, including the elimination of essential genes.
With a focus on improving integration efficiency, different strategies such as using repair templates with varying configurations, including classic HDR arms and tandem repeats, were tested. The precise amount of template DNA utilized was carefully adjusted for optimal outcomes.
Experiments also encompassed the examination of single-stranded DNA (ssDNA) donor templates, where integration rates were quantitatively assessed via flow cytometry to determine successful edits based on GFP expression levels.
Integration and Gene Tagging in Vivo in Xenopus
In the application of gene editing within living organisms, Xenopus tropicalis provides a valuable model due to its transparent embryos and rapid development. To optimize gene tagging and integration, specific gRNAs and repair templates were designed and injected into embryos.
Following a rigorous validation process involving the assembly of RNP complexes, embryonic injection protocols were refined. Post-injection, embryos were monitored, lysed, and subjected to sequencing analyses to confirm successful integrations.
The results were visually assessed using advanced imaging techniques, tracking the spatial expression of tagged constructs to understand the functional outcome.
Immunoprecipitation for Protein Characterization
To further explore gene function following editing, protein extraction from embryos was performed using immunoprecipitation methods. Embryos exhibiting successful expression of tagged proteins were harvested and subjected to specialized lysis protocols to ensure protein integrity.
Through proper immunoprecipitation and western blotting techniques, researchers sought to confirm the presence and functionality of the tagged proteins in developmental contexts, paving the way for dynamic studies of gene function.
µH Tandem Repeat-Mediated Integration in Mouse Brain
Expanding the utility of gene-editing techniques, procedures were adapted for use in mouse models. A combination of AAV vectors carrying the gRNA and Cas9 protein was injected into the mouse brain cortex via surgical methods under carefully monitored anesthesia.
Following a healing period, brain tissues were collected for further analysis, providing insights into endogenous gene expression post-editing. This multi-faceted approach is aimed at understanding gene function in the context of more complex organisms.
Advanced Imaging Techniques
The study of gene edits and biological processes relies heavily on advanced imaging techniques. For both Xenopus embryos and mouse brains, sophisticated imaging technologies such as mesoSPIM were employed to obtain high-resolution, three-dimensional views of developmental processes.
Through the use of specific fluorophores and imaging setups, these studies aimed to uncover the spatial dynamics of gene expression and protein localization within live organisms, contributing valuable information to the field of functional genomics.
DNA Preparation and Sequencing
Lastly, the preparation of DNA samples follows a regimented protocol tailored for diverse applications. Whether dealing with cell lines, embryonic lysates, or mouse brain tissues, the isolation method ensures quality samples for subsequent PCR and sequencing analyses.
The results from these sequencing efforts are analyzed to confirm successful integration events and assess the efficiency of gene editing, providing valuable feedback for future experiments.
Pythia In Silico Modeling
An innovative approach known as Pythia has emerged in the realm of CRISPR technology—this Python script simulates editing efficiencies using specific DNA sequences. By exploring multiple configurations of repair templates, Pythia identifies the most effective strategies for achieving precise genomic modifications.
Particularly relevant to clinical applications, Pythia’s modeling capabilities can guide researchers through complex genetic landscapes, shedding light on potential therapeutic strategies for disorders such as retinitis pigmentosa.
Conclusion
The exploration of cell culture methodologies and gene-editing technologies reveals a comprehensive workflow from basic laboratory practices to advanced experimental applications. Researchers continue to innovate in understanding gene function, repair mechanisms, and protein interactions, all while pushing the boundaries of genetic engineering.