Computational Methods for Cyclic Peptide Binder Design
Introduction
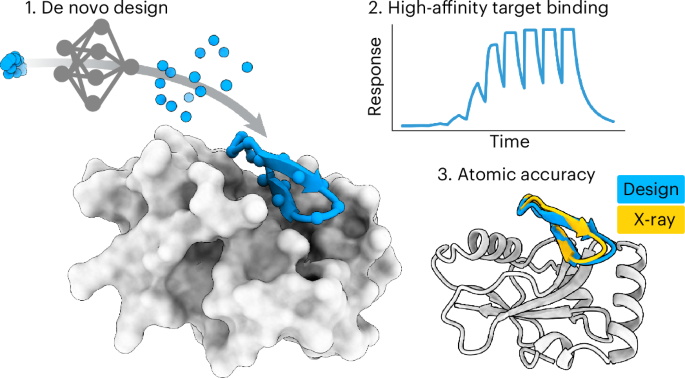
The design of cyclic peptide binders has become essential in drug discovery, particularly due to their favorable properties such as high stability, specificity, and affinity toward a variety of targets. This article delves into a detailed computational approach utilizing the RFpeptides framework for the design of macrocyclic peptide monomers and binders. The methodology implemented includes backbone generation using RFdiffusion, sequence design through ProteinMPNN, and structure prediction via AfCycDesign or RoseTTAFold, culminating in a robust pipeline for creating effective peptide binders.
Three-Stage Pipeline
1. Backbone Generation
The backbone generation of cyclic peptides is performed using RFdiffusion with a cyclic offset applied to peptide chains. RFdiffusion utilizes a novel diffusion process that efficiently samples potential conformational space of peptide backbones, generating diverse structures suitable for macrocyclic design. This step is crucial as the configuration significantly impacts the peptide’s ability to bind to targets effectively.
2. Sequence Design
Following the backbone generation, sequence design is accomplished via ProteinMPNN, a powerful neural network-based tool that generates amino acid sequences matched to the designed backbones. By leveraging previously known sequences and their structures, ProteinMPNN can predict sequences that could fold into stabilizing configurations, maximizing binding interactions with specific targets.
3. Structure Prediction
The final stage involves structure prediction of the designed peptide–target complexes. Here, tools like AfCycDesign or RoseTTAFold come into play, allowing for advanced structural modeling while applying cyclic offsets to preserve the macrocyclic nature of peptides. Predicting how these peptides interact with their intended targets ensures the designed binders exhibit the desired specificity and affinity.
Filtering and Downselection
After structure prediction, the designs undergo filtering based on Rosetta metrics—an evaluation encompassing factors like energy scores and folding potential. Additionally, in some cases, clustering based on Cα root mean square deviation (r.m.s.d.) is employed to prioritize designs with similar structural motifs, allowing further refinement of the peptide candidates.
Peptide Synthesis
Overview of Synthesis Strategies
The synthesized macrocyclic peptides in this study either stem from in-house production techniques or are acquired from commercial suppliers like Wuxi AppTec, ensuring purity levels exceed 90%.
Solid-Phase Peptide Synthesis (SPPS)
Peptides are synthesized using Fmoc-based solid-phase peptide synthesis. This robust methodology involves multiple steps:
- Resin Preparation: Broadly, CTC resin is preloaded and then swollen in DCM.
- Deprotection and Coupling: Iterative deprotection is achieved with 20% piperidine in DMF, followed by peptide elongation through coupling methods using HBTU or PyAOP supplemented with DIEA.
- Cyclization and Cleavage: The linear peptides are cleaved from the resin with either TFA in DCM or HFIP, followed by cyclization using solvents like DCM or DMF. The cyclization typically employs two equivalents of PyAOP and five equivalents of DIEA.
- Purification: The crude peptides undergo purification via reverse-phase high-performance liquid chromatography (HPLC), with identities confirmed by mass spectrometry.
Protein Expression and Purification
Expression of MDM2 and MCL1
For target proteins MDM2 and MCL1, their respective sequences are cloned into a Novogen pRSF-DUET plasmid that incorporates specific tags for ease of purification.
- Transformation of E. coli: Constructs are introduced into E. coli cells (NEBα for propagation, BL21(DE3) for expression).
- Induction and Harvesting: Cultures are induced with IPTG, subsequently harvested and lysed using a lysis buffer enriched with protease inhibitors to extract high-yield protein.
Purification Steps
Following lysis, proteins are purified using Ni-NTA affinity chromatography and further refined using size-exclusion chromatography (SEC). The purity and identity of the proteins are confirmed through SDS–PAGE and mass spectrometry, ensuring readiness for downstream applications.
Crystallization of Protein-Cyclic Peptide Complexes
Crystallization trials are conducted for complexes formed between target proteins and cyclic peptides. The sitting-drop vapor diffusion method is prominently employed:
- Complex Formation: Proteins and peptides are mixed in specific molar ratios and allowed to incubate, often followed by centrifugation to clear insoluble aspects.
- Setup of Crystallization Trials: Using robotic systems and various reservoir solutions, trials are established, leading to visible crystal growth.
Successful crystallization of complexes allows for X-ray structure determination, revealing insights into the interaction between cyclic peptides and their targets, thus facilitating further design iterations.
Surface Plasmon Resonance (SPR)
To evaluate the binding affinity of designed cyclic peptides against their targets, SPR assays are implemented. This technique provides real-time data on peptide interactions, allowing researchers to ascertain kinetic parameters effectively.
- Binding Kinetics: Biotinylated target proteins are immobilized, and binding studies are performed with varying peptide concentrations.
- Data Analysis: The resulting sensorgrams are analyzed using specialized software, producing affinity measures that guide further selection and refinement of peptide designs.
AlphaScreen Assay
For an alternative evaluation of cyclic peptide efficacy, AlphaScreen assays are utilized to measure inhibition of known interactions:
- Assay Setup: Biotin-labeled binder peptides are introduced alongside target components in a controlled environment to monitor competitive inhibition by designed cyclic peptides.
- Data Reporting: The results are normalized against controls for clarity, allowing determination of IC50 values that reflect potency and effectiveness in disrupting protein interactions.
By systematically applying these methodologies, researchers are equipped with a thorough toolkit for designing and characterizing cyclic peptide binders, pushing the boundaries of peptide-based therapeutics in biomedical research.
This organized approach serves not just to refine existing methodologies but also to pave the way for innovative solutions in drug discovery and development, enhancing our understanding of complex biological interactions at the molecular level.