A Comprehensive Overview of Radiomic Analysis in Myocardial Infarction Detection
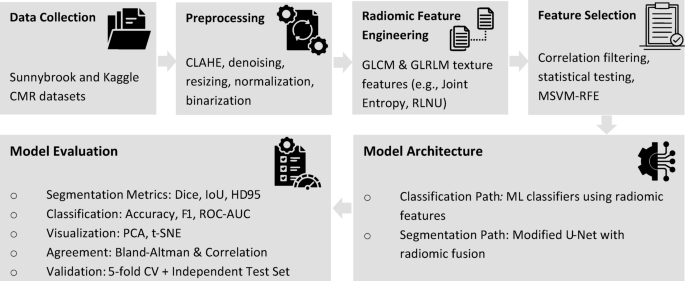
In the evolving field of medical image analysis, radiomics has emerged as a pivotal approach to enhance diagnostic accuracy in various conditions, particularly myocardial infarction (MI). This article delves into a structured overview of a model’s performance which encompasses radiomic feature extraction, segmentation models, classification evaluation, dimensionality reduction, and cross-validation techniques, giving insight into the model’s efficacy.
Radiomic Feature Extraction
Radiomic analysis is instrumental in uncovering detailed quantitative characteristics from medical imaging, transforming visual observations into measurable data. This analysis identified Joint Entropy as the standout discriminative feature, boasting an area under the curve (AUC) of 0.948 with a statistically significant p-value of (4.02 \times 10^{-13}) and a q-value of (2.01 \times 10^{-12}). The mean univariate AUC across all extracted features averaged around 0.73 ± 0.08.
A summary can be found in Table 3, which outlines key radiomic features following correlation filtering and statistical testing. This table includes AUC values, p-values, q-values, along with mean ± standard deviation for each feature, differentiating between MI and normal cases. Besides Joint Entropy, other noteworthy features like Max Probability (AUC = 0.885) and Run-Length Non-Uniformity (RLNU, AUC = 0.857) indicated statistically significant differences, particularly with Joint Entropy values being markedly higher in infarcted myocardium compared to normal tissue.
Visual Insights
Figure 3 presents boxplots of Z-score distributions for selected radiomic features. The visible differences between infarcted and healthy myocardium highlight the underlying heterogeneity within the radiomic data. Meanwhile, Figure 4 showcases radiomic texture feature maps derived from Gray Level Co-occurrence Matrix (GLCM), emphasizing the regional heterogeneity captured from cine-CMR images.
The first column displays the original cine-CMR slices for both normal and MI cases, while subsequent columns unveil computed maps for Joint Entropy, Max Probability, Sum Entropy, Joint Energy, and RLNU, underlining the visual complexity of infarcted regions compared to healthy tissue.
Univariate vs. Multivariate Feature Analysis
A comparative analysis was conducted between univariate and multivariate feature extraction methods for MI detection. The univariate approach, which focused on individual texture features, was juxtaposed with a multivariate method that noted higher-order descriptors such as autocorrelation. The results were compelling, with Table 4 revealing that classifiers utilizing the multivariate feature set consistently outperformed those with univariate features, indicating an enriched predictive capacity and robustness.
U-Net Segmentation Model
Building on the extracted radiomic features, the U-Net segmentation model was employed to pinpoint regions of myocardial infarction on late gadolinium enhancement cardiovascular magnetic resonance (LGE-CMR) images. Illustrated in Figure 6, the model’s performance displays a strong correlation between its predicted segmentation masks and expert annotations, although some discrepancies in boundary delineation are noted.
With a test set accuracy of 96.30%, the model demonstrated remarkable precision across both normal and MI classes. Detailed metrics were captured in Table 5, which highlights class-specific performance, revealing impressive precision, recall, and F1-scores for both normal and MI classifications.
Hybrid Segmentation Model: Integrating Radiomics
To further refine segmentation accuracy, radiomic features were incorporated into the U-Net model, creating a hybrid architecture. This fusion combined texture descriptors from GLCM and Gray Level Run Length Matrix (GLRLM), including parameters like autocorrelation and information correlation. Figure 7 displays this enhanced performance, with notable improvements observed in segmentation accuracy and interpretability compared to the baseline U-Net model.
An ablation study highlighted the benefits of data augmentation, as presented in Table 6, showing significant improvements in the Dice similarity coefficient and Intersection over Union (IoU).
Comparative Performance Metrics
When comparing segmentation performance, the hybrid U-Net model exhibited substantial improvements over its standalone counterpart, achieving a Dice score of 0.887 and a reduced 95th percentile Hausdorff Distance (HD95) of 4.48 millimeters. Metrics indicative of its enhanced accuracy are detailed in Table 7.
Dimensionality Reduction and Feature Visualization
Dimensionality reduction techniques, including PCA and t-SNE, were utilized to visualize the distribution of radiomic features, as depicted in Figure 9. PCA showed partial class separation between MI and normal cases, while t-SNE provided clearer clustering, reinforcing the notion that radiomic features are capable of effectively distinguishing between infarcted and healthy tissues.
Evaluation of Classification Performance
The classification performance of the hybrid U-Net model integrated with radiomic features was examined through various statistical analyses. Notably, Figure 10 presents a confusion matrix illustrating the model’s accuracy in distinguishing between normal and MI cases, achieving a remarkable AUC of 0.97, as showcased in Figure 11.
Further assessment using correlation and Bland-Altman analyses, displayed in Figure 12, demonstrated strong correlations between predicted and actual infarct sizes, affirming the model’s reliability and accuracy in infarct quantification.
Cross-Validation Performance
Finally, cross-validation efforts through a five-fold approach with both the Sunnybrook Cardiac Dataset and Kaggle dataset confirmed the model’s robustness and generalizability, with fold-wise accuracy consistently reflecting above 96%. Figure 13 illustrates the individual fold accuracies, emphasizing the model’s consistent performance across different data partitions.
This structured overview reflects the promising potential of combining radiomic analyses with advanced machine learning techniques in accurately diagnosing and segmenting myocardial infarction, ultimately paving the way for improved clinical outcomes in cardiac care.