Ethical Approval and the Development of the DeepRETStroke System
Ethical Approval
Before the inception of a study that aligns artificial intelligence with health outcomes, obtaining ethical approval is paramount. The DeepRETStroke system, designed to innovate in stroke detection and prediction through retinal imaging, received formal approval from the Ethics Committee of Shanghai Sixth People’s Hospital (approval no. 2023-KY-023 [K]). Notably, the foundational development and validation phases of the DeepRETStroke system utilized deidentified retrospective data, freeing the study from necessitating active participant involvement. However, in the real-world prospective study, informed consent was acquired from all participants, in strict adherence to the tenets of the Declaration of Helsinki. This commitment to ethical standards ensures that the system’s implementation safeguards participant rights while contributing to important medical advancements.
Study Sample
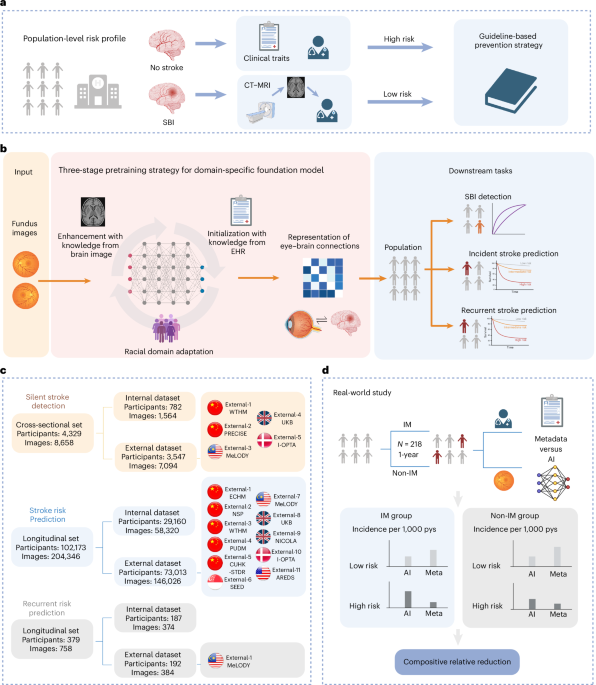
The primary aim of the DeepRETStroke system was to harness retinal images for detecting silent brain infarcts (SBI) and predicting incident strokes. The model was meticulously fine-tuned for the prediction of recurrent strokes as well. For the SBI detection phase, retinal photographs from six independent datasets were utilized. The inclusion criteria were strictly defined, accepting only those participants who had undergone retinal photography and brain MRI or CT scans, without any previous history of overt strokes. Similarly, for predicting incident strokes, retinal photographs from twelve independent datasets formed the backbone of the model’s validation, again prioritizing participants devoid of a stroke history at baseline. The recurrent stroke prediction component relied on retinal images from two independent datasets focused on those with established stroke histories. Notably, meticulous filtration of image-level data was applied, ensuring no overlap between developmental and validation sets, allowing for robust model validation.
Image Quality Control
Image quality is paramount when dealing with retinal photography; factors like clarity can significantly affect predictive accuracy. The retinal images for this study were captured using renowned standard fundus cameras, such as the Topcon TRC-NW6 and Canon CR1–Mark II. The quality of these images was rigorously assessed following criteria defined by Carol et al., which dictated exclusion for images where over 25% of the peripheral retina was unobservable or if substantial artifacts were present in the central region. Post quality control, these fundus images were handed over to the artificial intelligence team, laying the groundwork for the DeepRETStroke system.
Fundus Image Enhancement
In order to capture the vascular features relevant to systemic diseases, a systematic approach to image enhancement was employed. Initially, a technique known as contrast-limited adaptive histogram equalization boosted image contrast while minimizing noise interference. This process involved transforming the fundus images from RGB to LAB color space, segmenting them into fixed-size pieces, and applying the enhancement to each piece individually before reverting to RGB. Subsequently, colour normalization was introduced to counteract variability arising from different photographic conditions. The enhanced images were then resized to a resolution of 512×512 pixels, ensuring consistency for subsequent analytic phases.
Definition and Criteria for Disease Diagnosis
Defining silent brain infarcts (SBI) formed the basis for accurate clinical diagnoses. In this study, the determination hinged on clinical criteria comprising physician-diagnosed cerebral infarcts visible in CT or MRI scans, but without any self-reported history of strokes. The criteria for incidents and recurrent strokes were similarly robust, relying on guidelines set forth by the American Heart Association and the American Stroke Association. This meticulous definition ensured that all diagnoses adhered to established medical standards, lending credence to the research findings.
Development of the DeepRETStroke System
The architecture of the DeepRETStroke system represents a cutting-edge integration of domain-specific knowledge targeting eye-brain connections. Its development unfurled through a three-stage pretraining strategy. Initially, the RETFound model served as the fundamental encoder, followed by a phase utilizing the Masked AutoEncoder to enhance generalizability across diverse racial cohorts. In the subsequent stage, a model initialization process addressed cold starts, building a foundational understanding of the eye-brain connections before advancing further.
The third stage revolved around iterative training, embracing semi-supervised learning and knowledge transfer, allowing the system to refine its predictive accuracy dynamically. This structured training not only optimized the encoder’s performance but also equipped it with robust knowledge about cerebrovascular conditions, paving the way for an effective stroke prediction model.
Development of the Metadata Model and Combined Model
To sufficiently evaluate the DeepRETStroke system’s efficacy, supplementary models were crafted. For SBI detection, a metadata model was introduced, employing logistic regression with a suite of cardiovascular risk factors like age, BMI, and cholesterol levels. Similarly, for incident and recurrent stroke predictions, a Cox-proportional hazards model was devised using the same foundational risk factors, serving to augment the predictive framework established by the DeepRETStroke system.
Implementation Details of the DeepRETStroke System Developmental Process
The developmental journey was anchored in utilizing the retinal feature encoder from RETFound as the backbone of the DeepRETStroke system. It involved comprehensive steps, from selecting the right model to applying advanced training techniques like data augmentation and batch training, utilizing tools such as the Adam optimizer. These detailed methodologies ensured the model remained robust, reliable, and ready for real-world application.
Real-World Study of the DeepRETStroke System
A real-world application of the DeepRETStroke system unfolded within a community-based prospective cohort study involving Chinese adults. This study screened a total of 218 participants, gathering crucial baseline data and assessing metabolic factors, ultimately integrating this practical application to validate theoretical findings.
Explainability Analysis of the DeepRETStroke System
Understanding the decision-making processes of machine learning models is vital, especially in health applications. For the DeepRETStroke system, explainability was achieved using techniques like GradientShap and the occlusion method. These strategies allowed researchers to visualize which areas of the retinal images most influenced the model’s predictions, thereby enhancing trust and transparency in the system’s outputs.
Statistical Analysis
Statistical methods played an essential role in evaluating the performance metrics of the DeepRETStroke system. Deploying techniques like ROC curve analysis, sensitivity estimation, and Harrell’s C-index ensured a comprehensive understanding of the system’s predictive validity. Such rigorous analysis underscored the importance of employing established statistical frameworks to bolster findings derived from the DeepRETStroke system.
In sum, this meticulous journey from ethical approval to predictive modeling encapsulates the potential that cutting-edge AI approaches have in enhancing our understanding of cerebrovascular health through the lens of retinal imaging. Each stage of the development offers invaluable insights not only into the technological advancements of healthcare innovations but also into the ethical frameworks guiding such research pursuits.