HoVer-NeXt: Enhancing Identification of Immune Cells in Histopathology
Accurate Cell Detection with HoVer-NeXt
The advent of sophisticated machine learning tools has revolutionized pathology, particularly through innovative initiatives like HoVer-NeXt. This advanced nuclei segmentation and classification model has demonstrated outstanding performance in accurately identifying key immune cell types—lymphocytes, eosinophils, and intraepithelial lymphocytes (IELs)—in hematoxylin and eosin (H&E) whole slide images (WSI). Previous validations highlighted an impressive F1 score of 0.77 for lymphocyte detection and 0.83 for epithelial cells, affirming the model’s efficacy in this critical domain.
Eosinophils, while vital to immune surveillance, are markedly less common than lymphocytes and epithelial cells. To ensure accurate detection of this cell type, researchers created a meticulously annotated test set comprising eleven regions of interest (ROIs) from seven WSI, encompassing a staggering 4,952 eosinophils with visible nuclei. The model achieved an F1 score of 0.73 ± 0.07, with a precision of 0.71 ± 0.12 and recall of 0.78 ± 0.07. Additionally, IELs also underwent validation within the same ROIs, yielding an F1 score of 0.407 ± 0.061 and a remarkable ROI-level correlation of ρ = 0.87. This dataset provides a crucial tool for further research and is publicly available for interested researchers.
Understanding Eosinophils and IELs in the Tumor Immune Microenvironment
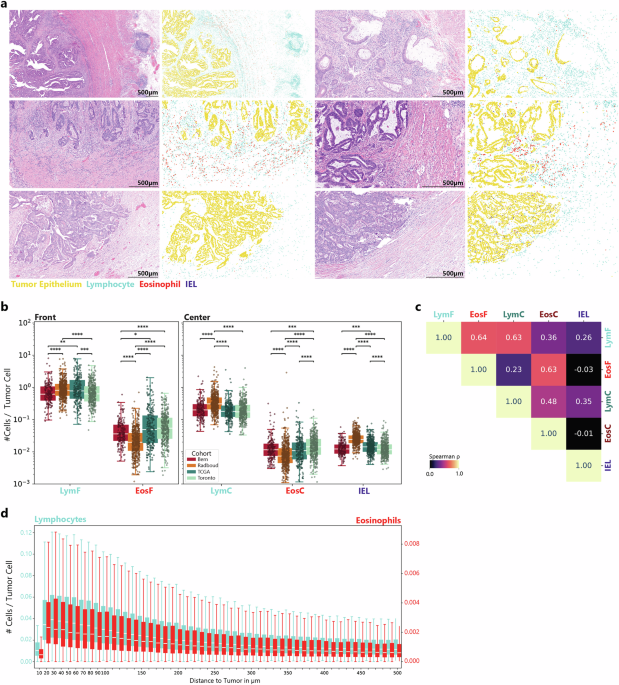
In this study, researchers meticulously examined the distribution and correlation of eosinophils, lymphocytes, and IELs to unravel their interrelationship within the tumor microenvironment. Exploratory analyses revealed that, on average, the tumor front houses more eosinophils and lymphocytes (EosF: 6.02% ± 4.49%; LymF) compared to the tumor center (EosC: 5.72% ± 3.93%). Notably, scores were normalized per cohort using a percentile approach for robustness against outliers, particularly in cases with a lower tumor cell count.
This correlation analysis revealed a strong positive correlation between eosinophils and lymphocytes at the tumor front (ρ = 0.64), as well as within the tumor center (ρ = 0.63). In contrast, IELs demonstrated weaker correlations with lymphocytes and eosinophils, indicating their unique role in immune response dynamics within the tumor.
Eosinophils as Independent Prognostic Indicators
The study further aimed to explore the prognostic significance of these immune populations by correlating their presence with various clinicopathological characteristics. Eosinophils and IELs exhibited a notable relationship with favorable prognostic factors, particularly in stage II cases. Eosinophils and IELs were significantly more abundant in tumors without invasive characteristics, suggesting their potential protective role in tumor biology.
When evaluated through univariate survival analysis, increased counts of LymF, EosF, and IELs were associated with markers of a more favorable prognosis. In patients with microsatellite instability (MSI), low eosinophil counts correlated with poorer survival, while increased counts in mismatch repair proficient (MSS) cases indicated a positive prognostic effect.
Patterns of Immune Infiltration and Its Implications
An intriguing aspect of this research was the exploration of immune infiltration patterns in relation to tumor subtypes. The study investigated various microsatellite instability states and molecular subtypes of colorectal cancer (CRC). Eosinophils surfaced as noteworthy players in MSI cases, marked by increased abundance alongside lymphocytes and IELs. However, they did not demonstrate a consistent relationship with molecular subtype classifications such as Consensus Molecular Subtypes (CMS) or Pathway Discovery Subgroups (PDS), emphasizing the unique role they play in tumor immunity.
Furthermore, the relation between immune cell groups and established inflammation markers, such as the Klintrup-Mäkinen score, posited a link between eosinophil presence and tumor biology, indicative of a broader immune response paradigm driven by distinct molecular processes.
Additive Prognostic Value of Immune Cell Types
Upon conducting multivariate survival analyses, it was evident that eosinophils and IELs maintained their independent prognostic significance. In a model that considered factors such as age, sex, tumor location, and treatment parameters, higher levels of these immune cell types continued to correlate with improved outcomes across various stages of CRC.
Notably, the combined presence of eosinophils and IELs provided the best model fit based on Akaike Information Criterion (AIC) values. This finding underscores their potential additive value in prognostic assessments, particularly within certain patient subgroups.
In summary, the critical insights gained through this study not only elucidate the vital role of specific immune cell populations in colorectal cancer pathology but also highlight the necessity of integrating advanced computational tools like HoVer-NeXt into routine histopathological assessments for enhanced prognostic outcomes. These findings pave the way for future research and clinical application, potentially refining therapeutic strategies centered around the tumor immune microenvironment.