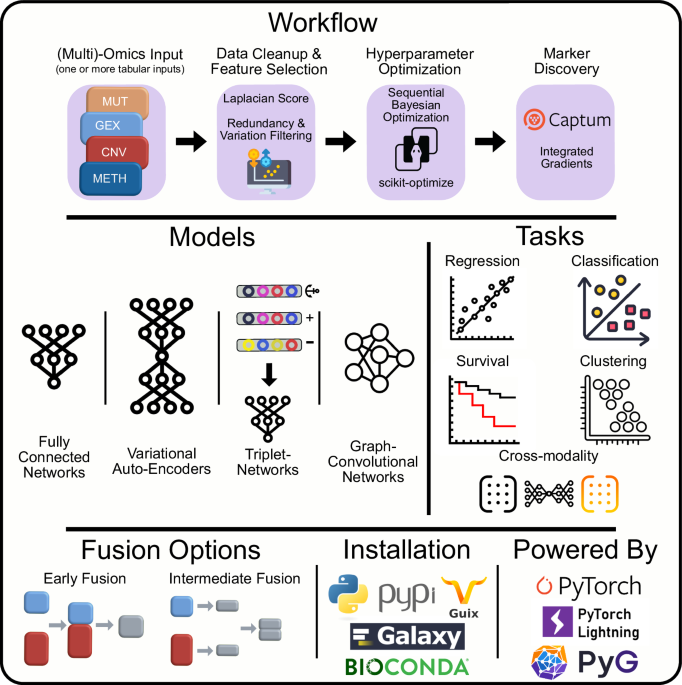
Flexynesis: Harnessing the Power of Predictive Modeling in Automated Construction
Flexynesis is a revolutionary platform designed for the automated construction of predictive models targeting one or more outcome variables. This powerful system integrates advanced machine learning techniques, notably the supervisor multi-layer perceptron (MLP), to connect with various encoding networks—comprising both fully connected and graph-convolutional architectures. This design allows for tailored predictive modeling for clinically relevant tasks, including drug response prediction, disease subtype classification, and survival analysis. Flexynesis effectively reveals how each outcome variable influences low-dimensional sample embeddings—the latent variables resulting from the encoding networks, thereby optimizing predictive accuracy.
Single-task Modeling: Focused Predictions
Single-task modeling with Flexynesis offers a comprehensive approach to prediction by enabling models to focus on one specific outcome variable at a time. Various modeling tasks are achievable, including regression, classification, and survival models.
For instance, in drug response prediction, Flexynesis utilizes multi-omics data, such as gene expression and copy-number variation, from established databases like the Cancer Cell Line Encyclopedia (CCLE). By training on this data, Flexynesis generates insightful predictions about cell line sensitivity to specific drugs, such as Lapatinib and Selumetinib. The predictive model demonstrates high correlation values with known drug responses—from the Genomics of Drug Sensitivity in Cancer (GDSC2) database—highlighting its effectiveness and reliability.
Similarly, for classification tasks, Flexynesis successfully predicts microsatellite instability (MSI) status across diverse cancer types using gene expression and promoter methylation profiles. This application is particularly notable as MSI status is crucial for determining treatment options in immunotherapy. The model achieves impressive classification accuracy, illustrating how it can discern critical therapeutic insights even in the absence of genomic sequencing data.
Survival modeling adds another layer of complexity, as demonstrated through data from lower-grade glioma and glioblastoma cohorts. By implementing a supervised MLP tailored to predict patient-specific risk scores, Flexynesis stratifies patients effectively based on predicted survival outcomes, demonstrating significant potential in clinical decision-making.
Multi-task Modeling: Enhancing Predictive Flexibility
Flexynesis’s true strength shines in multi-task modeling, which allows simultaneous predictions of multiple outcome variables. Here, multiple MLPs are affixed atop the encoding networks, enhancing the model’s ability to mold embeddings from several clinically relevant variables, even in situations where some labels may be missing.
Using the METABRIC dataset, which comprises profiles from metastatic breast cancer patients, Flexynesis showcases its prowess by training on clinical variables like breast cancer subtype and chemotherapy treatment status. The results indicate that the multi-task model adeptly captures the relationships between both variables, unlike single-task approaches which may falter in representing concurrent influences. This ability to integrate diverse clinical information concurrently lays a transformative groundwork for future applications in precision medicine.
Unsupervised Learning: Discovering Patterns
The versatility of Flexynesis is further exemplified through its implementation of unsupervised learning techniques, specifically utilizing variational autoencoders (VAEs) with maximum mean discrepancy (MMD) loss. The model can autonomously discover underlying patterns in cancer data, grouping samples based on inherent characteristics without predefined labels.
In a pilot test involving gene expression and methylation data from 21 cancer types, Flexynesis employs k-means clustering to categorize samples distinctly. The resulting t-SNE visualizations illustrate a robust correspondence between the unsupervised model’s clusters and known sample labels, further validating the VAE’s capability in understanding complex biological data structurally.
Cross-modality Learning: Bridging Diverse Data Types
One of the features that sets Flexynesis apart is its ability to facilitate cross-modality learning, enabling the model to learn from and translate across differing data modalities. This innovative approach allows gene expression data to reconstruct mutation data, enhancing the model’s understanding of the relationship between diverse biological interactions.
A practical illustration of this methodology involves employing gene expression profiles alongside protein sequence embeddings generated by large language models and structural features from dedicated databases. The model not only predicts gene essentiality scores in cell lines but simultaneously encodes essentiality-related features into a consolidated framework, offering insights that could be pivotal in cancer research and treatment strategies.
Model Fine-tuning: Enhancing Adaptability
Flexynesis provides an optional fine-tuning procedure that refines models trained on a source dataset by allowing them to adapt to the peculiarities of a target dataset. This feature is crucial when datasets originate from different sources with potentially varying distributions, improving model accuracy and reliability in real-world applications.
Initial experiments highlight the benefits of fine-tuning when transitioning from train data (such as CCLE) to test data (such as GDSC), allowing models to adjust dynamically and enhance performance metrics significantly. This adaptability further emphasizes Flexynesis as a solution that stands at the confluence of classical machine learning techniques and advanced deep learning models.
Discovering Biomarkers of Drug Response
A distinctive capability of the Flexynesis framework is its integrated marker discovery module that utilizes feature attribution methods like Integrated Gradients and GradientSHAP. In examining prediction tasks for drug response, the model identifies biomarkers associated with known molecular targets, facilitating deeper investigations into treatment responsiveness.
Through its systematic approach, Flexynesis not only validates existing knowledge regarding drug markers but also reveals new actionable insights. The integration of varied genomic layers strengthens predictions, aligning with findings that underscore the importance of combining multiple data modalities for optimal outcomes.
The Flexynesis Benchmarking Pipeline: Ensuring Optimal Model Selection
Given the complexity and diversity of available algorithms, model selection stands as a crucial challenge in predictive modeling. The Flexynesis benchmarking pipeline tackles this head-on, allowing users to experiment with various configurations across different data modalities, fusion methods, and model architectures efficiently.
This organized experimentation framework facilitates 222 unique trials across various datasets, yielding a rich dashboard that ranks model performance across multiple clinical prediction tasks. The findings underscore the importance of flexible modeling strategies, where neither deep learning nor classical methods consistently outperform each other, suggesting that both approaches should be evaluated contextually.
By leveraging this comprehensive pipeline, researchers and clinicians can iteratively refine their modeling strategies, ensuring that they derive the most effective insights from their datasets, regardless of the complexity involved.
Through all these features, Flexynesis emerges as a transformative tool in predictive modeling that seamlessly integrates clinical relevance with cutting-edge machine learning techniques, enhancing the potential for breakthroughs in patient care and personalized medicine.