Deep-Learning-Guided Identification of Archaeasins: A Revolutionary Approach in Antimicrobial Research
Introduction to Archaeasins
In the quest for new antimicrobial agents, researchers have turned their focus toward archaea, a group of single-celled microorganisms that thrive in extreme environments. Recently, deep learning models have emerged as powerful tools for sifting through vast biological data, enabling scientists to identify potential antimicrobial peptides (AMPs) within archaeal proteomes. This article delves into a groundbreaking study that employed deep learning to identify archaeasins—antimicrobial peptides derived from archaea—highlighting the methodology, findings, and implications for addressing antibiotic resistance.
Methodology: Utilizing APEX 1.1 for Antimicrobial Prediction
In this study, researchers began by collecting 18,677 non-redundant reviewed protein sequences from 233 archaeal organisms available on UniProt. To identify antimicrobial peptides, they employed APEX 1.1, a deep learning antimicrobial activity predictor, retrained on updated data. This model predicted minimum inhibitory concentrations (MICs) specific to various bacterial strains, allowing the researchers to gauge the overall antimicrobial potency of the peptides.
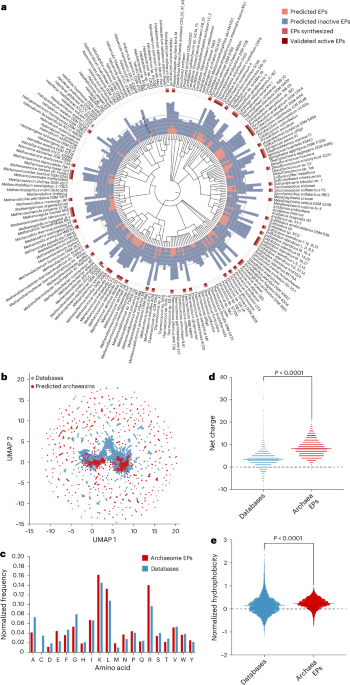
Through this systematic scan of archaeal proteomes, the researchers identified 12,623 antimicrobial peptides with a mean MIC of ≤100 μmol/l, representing about 0.00653% of the total 193,331,608 archaeal peptides analyzed. To establish whether this finding was statistically significant, a non-redundant set of 193,288,387 randomly sampled peptides was generated, yielding an AMP rate of just 0.00274%. This stark contrast indicated that antimicrobial sequences are indeed enriched within archaeal proteomes, marking a crucial finding for future antimicrobial research.
Sequence and Structural Analysis of Archaeasins
A deeper exploration of the identified archaeasins’ sequence space used UMAP (Uniform Manifold Approximation and Projection) to visualize peptide similarities. The researchers compared the amino acid frequency of archaeasins against known AMPs from existing databases, revealing distinct compositional features. Notably, archaeasins exhibited an enrichment of glutamic acid residues, leading to a unique balance in charge distribution. This distribution is essential as it influences how peptides interact with bacterial membranes.
Moreover, investigating physicochemical properties, researchers noted that while the hydrophobicity of archaeasins was comparable to other AMPs, they exhibited a tendency toward increased amphiphilicity, suggesting a strategic design for targeting bacterial membranes.
Genomic Insights: Correlation Between Genome Size and AMP Prevalence
The study also raised intriguing questions about the relationship between genome size and the number of predicted antimicrobial peptides. By analyzing the top 10 archaeal genera with the highest and lowest EP counts, researchers discovered a statistically significant positive correlation. Larger archaeal genomes tended to encode more predicted AMPs, suggesting that these organisms may harbor a broader spectrum of latent antimicrobial sequences.
Interestingly, many genera that were top scorers, such as Pyrococcus and Methanocaldococcus, are known for their thermophilic lifestyles. This observation implies that environmental factors, particularly temperature tolerance, may play a pivotal role in the evolution of antimicrobial sequences.
Experimental Validation of Antimicrobial Activity
To experimentally validate the predictive power of APEX 1.1, researchers synthesized 80 of the top-ranking archaeasins for testing against various clinically relevant pathogens, such as Acinetobacter baumannii and Staphylococcus aureus. Remarkably, 75 out of 80 archaeasins demonstrated antimicrobial activity (MIC ≤ 64 μmol/l) against at least one bacterial strain, yielding a hit rate of over 93%. This validation not only confirmed the accuracy of the predictive model but also showcased the potential of archaeasins as candidates for combating multidrug-resistant infections.
Secondary Structure and Mode of Action
Understanding the secondary structure of these peptides is critical for determining their mechanism of action. Using circular dichroism experiments, researchers found that archaeasins primarily adopted disordered conformations in aqueous environments. However, in media that favored helical structures, such as TFE-water mixtures, they transitioned towards more structured forms. Such dynamics are vital for effective membrane interaction.
Fluorescence assays assessed the membrane-targeting capabilities of the archaeasins, revealing that many peptides effectively permeabilized the outer membrane of A. baumannii, while others were more efficient at depolarizing the cytoplasmic membrane. This indicates that archaeasins primarily exert their effects by disrupting membrane potential—a mechanism that is both innovative and essential for the development of new antimicrobial agents.
Investigating Synergistic Interactions
The study also explored whether combinations of archaeasins could synergize or enhance each other’s antimicrobial activities. Checkerboard assays revealed a variety of interactions, with many pairs showing synergistic or additive effects. Such combinations can lead to enhanced efficacy while also potentially reducing toxicity and resistance development. Notably, compounds from hyperthermophilic species, particularly Methanocaldococcus and Thermococcus, displayed particularly strong synergistic interactions, suggesting an evolutionary advantage in high-temperature environments.
Toxicity Profiles of Archaeasins
Evaluating the potential toxicity of these peptides is crucial for therapeutic applications. Among the 80 archaeasins tested, many displayed low to moderate hemolytic activity against human red blood cells and low cytotoxicity in human cell lines. This is an encouraging aspect of archaeasins, as it suggests they can retain their antimicrobial potency while minimizing off-target effects.
Preclinical Evaluation: Efficacy in Animal Models
To further assess their practical applications, archaeasins were tested in mouse models for skin abscess and deep thigh infections. The peptides showcased significant antimicrobial activity against A. baumannii, demonstrating their potential as effective treatment options in complex biological systems. Importantly, these experiments indicated that archaeasins not only hinder bacterial growth but also exhibit low toxicity, reinforcing their viability as antimicrobial agents for future development.
In summary, this study highlights the utility of machine learning in discovering novel antimicrobial peptides from archaea. The findings suggest a promising future for archaeasins as effective antimicrobial agents, particularly in combating resistant bacterial infections, and warrant further exploration within the broader context of antimicrobial research and development.