Quantum Machine Learning Techniques in Predicting Drug Sensitivity in Multiple Myeloma
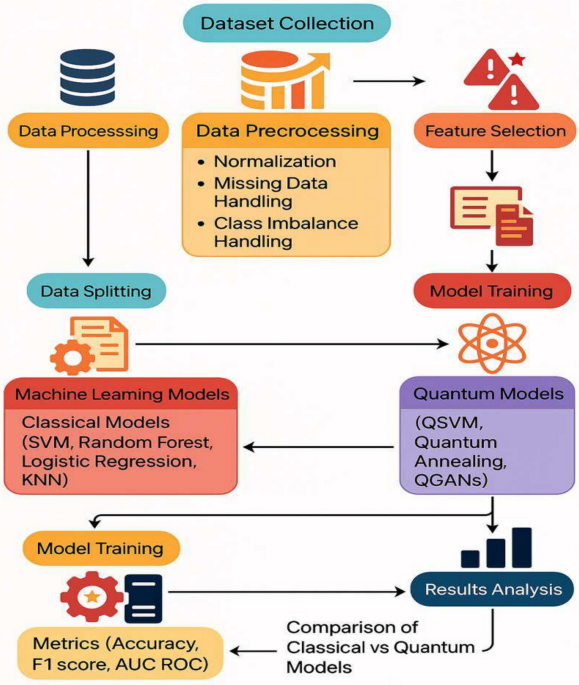
The scientific community is increasingly exploring the intersection of quantum computing and machine learning, particularly in the field of oncology, where deciphering complex datasets can lead to significant advancements in treatment personalization. This article dives into a study utilizing a proteomic dataset from 39 Multiple Myeloma (MM) patients, focusing on how quantum machine learning (QML) methodologies can improve predictions of drug sensitivity, ultimately shaping more effective therapeutic strategies.
Dataset Description
The dataset, titled proteomics_data.csv, encompasses protein expression profiles for 2573 proteins across 39 patients diagnosed with Multiple Myeloma. The dataset was derived using an Individualized Systems Medicine approach, specifically via mass spectrometry analysis of CD138+ plasma cells. This technique stratifies MM patients into four chemosensitivity categories: high sensitivity, sensitivity, resistance, and high resistance, based on their response to treatment.
Importantly, ethical implications were minimal, as this study utilized simulated data, eliminating the requirement for ethical approval or informed consent. Researchers examined six clinically relevant drugs, calculating drug sensitivity scores (DSS), which were transformed into binary labels: sensitive (DSS ≥ 10.0) or resistant (DSS < 10.0). This binary classification aligns with previously established thresholds in drug sensitivity testing.
The dataset’s format is depicted mathematically as:
[
X = { x{1}, x{2}, \ldots, x{n} }
]
Here, each ( x{i} ) denotes a protein expression vector in ( R^{p} )—where ( p ) represents the number of protein features (2573) for each patient.
Notably, the dataset confronts challenges typical of biomedical research, including:
- High Dimensionality: The feature dimension exceeds the sample size (( p \gg n )).
- Class Imbalance: Approximately 83% of patients are drug-sensitive, while only 17% are drug-resistant.
These challenges can lead to overfitting in traditional machine learning scenarios, necessitating advanced methods to ensure robust predictions.
Data Preprocessing
Prior to analysis, several preprocessing strategies were employed to prepare the dataset effectively for classical and quantum machine learning models:
Normalization of Protein Expression Values
The normalization of raw protein expression levels was critical due to significant variations arising from experimental and biological factors. Max-min normalization was applied to rescale values to a [0, 1] range:
[
x’ = \frac{x – \text{min}(x)}{\text{max}(x) – \text{min}(x)}
]
This standardization ensures that variations in experimental conditions do not disproportionately influence model outputs.
Dimensionality Reduction and Feature Selection
The high dimensionality of the dataset necessitated dimensionality reduction techniques such as Principal Component Analysis (PCA) and its quantum counterpart, Quantum Principal Component Analysis (qPCA). The goal of these methods is to retain maximum variance in a reduced number of features, thus enhancing model efficiency.
Analysis of Variance (ANOVA) F-Statistic
To assess the discriminative power of individual proteins regarding drug sensitivity, the ANOVA F-statistic was employed. This approach helps identify the most relevant features for predicting sensitivity, ranking them based on their ability to differentiate between sensitive and resistant patients.
Minimal-Redundancy, Maximal-Relevance (mRMR)
To address redundancy among highly correlated features, the mRMR feature selection methodology was utilized. This strategy finds a subset of features that are not only relevant to the target variable (drug sensitivity) but also minimize redundancy amongst themselves.
Addressing Class Imbalance
With a highly imbalanced class distribution in the dataset, statistical techniques such as stratified sampling during cross-validation were implemented, ensuring that both sensitive and resistant classes were adequately represented in training and testing sets. Additionally, class-weighted loss functions were introduced to penalize misclassification of the minority class.
Five-Fold Cross-Validation
To mitigate overfitting, five-fold cross-validation was employed. The data is partitioned into five subsets, allowing each subset to serve as a test set while the remaining four subsets form the training set. This practice enhances the robustness of the model when applied to unseen data.
Quantum Machine Learning Methods
The study extensively employed various Quantum Machine Learning techniques to tackle the challenges of dimensionality, class imbalance, and feature redundancy.
Quantum Support Vector Machines (QSVM)
QSVM extends classical Support Vector Machines to higher-dimensional Hilbert spaces using quantum kernel methods. This advantage enables QSVM to capture nonlinear relationships among features more effectively than traditional SVMs. By employing quantum kernels, accuracy in classifying drug sensitivity improves significantly amid imbalanced datasets.
Quantum Principal Component Analysis (qPCA)
Applying qPCA aims to retain significant variance in the data while reducing dimensionality. Utilizing quantum circuits allows for more efficient computations, capturing crucial patterns while lessening the feature space. This transformation helps simplify the dataset, making subsequent machine learning tasks more manageable.
Quantum Annealing for Feature Selection
Quantum Annealing optimization was utilized to curate the best proteins for predicting drug sensitivity. By minimizing the selection of features while maintaining prediction accuracy, Quantum Annealing strategically narrows down from a larger feature set to only those proteins most relevant for classifying drug response.
Quantum Generative Adversarial Networks (QGANs)
To address class imbalance effectively, QGANs were employed. This approach generates synthetic data representing the minority class (drug-resistant patients). The QGAN architecture includes a quantum generator and a classical discriminator that competes against each other, ultimately producing high-quality synthetic data that augments the original dataset.
Conclusion
The integration of quantum machine learning techniques with proteomic data presents a groundbreaking opportunity to improve the prediction of drug sensitivity in Multiple Myeloma patients. These methods not only address prominent challenges such as high dimensionality and class imbalance but also pave the way for advancements in personalized medicine and treatment efficacy.
The ongoing exploration of QML in oncology is poised to revolutionize therapeutic strategies, making significant strides toward enhancing patient outcomes and redefining treatment protocols in the future.