Advancements in Histological Image Analysis: Semantic Segmentation of Mouse Lung Tissue
In recent years, histological imaging has become a pivotal tool in biomedical research, particularly for understanding lung biology and pathology. In this article, we delve into a fascinating study that explores the preprocessing, annotation, and segmentation of raw histological images from mouse lungs subjected to various cell seeding methods. This research illustrates the intricate interplay between experimental techniques and sophisticated machine learning models like U-Net and LinkNet, aimed at enhancing our understanding of lung tissue re-endothelialization.
Obtaining and Preprocessing Histological Images
The histological images used in this study were meticulously sourced from the work by Chan et al. featuring male C57BL/6 J strain mice aged between 12 to 14 weeks. The euthanization process was performed humanely through CO2 exposure, leading to the ex vivo cell seeding using a phosphate-buffered saline (PBS) injection into the right ventricle. The heart-lung block was subsequently removed and preserved for decellularization using established protocols. This process effectively removes cellular components, leaving behind a scaffold that can be repopulated with endothelial cells.
The re-endothelialization was performed with C166 cells encapsulated in a specially prepared medium, and various seeding techniques were applied, including direct injections, negative pressure, and a novel dual pressure approach. Each method aimed to optimize cell distribution throughout the lung scaffold, creating a robust platform for subsequent imaging and analysis.
Annotation Techniques and Image Processing
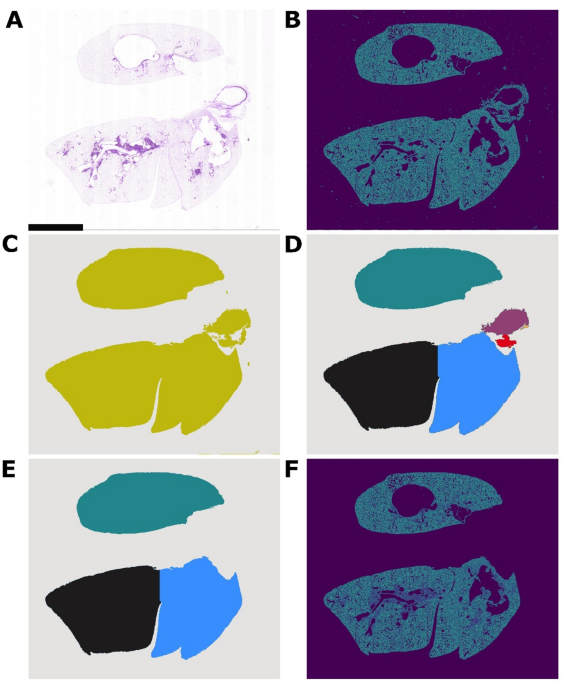
A crucial step in analyzing histological images is annotation, which plays a foundational role in training machine learning models. The study employed a comprehensive image preprocessing pipeline that began with Vahadane stain normalization—a technique designed to mitigate color variability across images stemming from different staining techniques.
The normalization was followed by a Canny edge detection algorithm to sharpen the boundaries within the images, followed by advanced techniques like watershed segmentation to ensure that overlapping heart tissue did not confound the analysis of lung cells. The entire process utilized a series of morphological operations to enhance image clarity and precision.
After creating masks that accurately delineated lung scaffold and seeded cell regions, the researchers employed multi-Otsu thresholding to classify pixels into distinct categories: background, lung scaffold, and seeded cells. This method allowed for a more systematic and dependable segmentation process, minimizing the biases inherent in lengthy manual annotation methods.
Model Training with U-Net and LinkNet
The study harnessed the power of two prominent convolutional neural network (CNN) architectures—U-Net and LinkNet—to perform semantic segmentation on the preprocessed images. Both models are designed to excel with high-resolution images, especially in scenarios with limited sample sizes. U-Net is characterized by its encoder-decoder structure that captures both contextual and spatial information, making it particularly effective for biomedical image segmentation. LinkNet, on the other hand, requires fewer parameters and less training time while still maintaining high accuracy—a vital aspect considering the relatively small dataset.
To maximize the training data effectiveness, patch-based learning techniques were implemented. This approach involved breaking down larger images into smaller patches, significantly increasing the amount of training data available while preserving important details that may otherwise be lost through resizing.
Leveraging Transfer Learning and Data Augmentation
To further bolster model performance, transfer learning was employed, benefiting from models pre-trained on extensive datasets such as ImageNet. By utilizing the established weights from these models, the researchers could fine-tune the network to better adapt to their specific histological imaging tasks.
Data augmentation techniques were also applied to introduce variability into the training set, allowing the model to become more robust against changes in orientation and scale. These techniques ultimately help in reducing overfitting, which is particularly crucial when dealing with limited datasets.
Performance Metrics: Precision, Recall, and Accuracy
To evaluate the success of the semantic segmentation models, various classification metrics were calculated, including precision, recall, and the Intersection over Union (IoU). Precision measures the proportion of true positive predictions against all positive classifications made by the model, while recall quantifies the model’s capability to accurately identify all positive instances present in the dataset.
In addition to these metrics, the study also calculated the root mean square error (RMSE) for the Cell Seeding Classification (CSC) predictions, serving as a robust indicator of the model’s accuracy in predicting seeded cell areas relative to ground truth values.
Challenges and Considerations in Model Evaluation
Despite the well-structured methodology, challenges persisted in model training and evaluation. With a limited number of images, the efficacy of segmentation could vary widely. Implementing thorough cross-validation techniques helped in assessing the reliability of model performance, ensuring that variations were accounted for and that results were generalizable.
This study represents a significant step forward in the realm of histological image analysis, combining sophisticated biotechnological approaches with cutting-edge machine learning techniques. By refining segmentation processes and improving training methodologies, researchers can pave the way for more accurate diagnostics and therapeutic strategies in lung health and disease.
These innovations not only enhance our understanding of lung re-endothelialization but also foster the development of machine learning in histopathology, presenting exciting opportunities for future research and clinical applications.