Exploring Animal Models and Cell Culture Techniques in Biomedical Research
In biomedical research, understanding the mechanisms of diseases and potential treatments often requires the careful use of animal models and advanced cell culture techniques. This article delves into various methodologies utilized to study cellular responses and their implications in broader biological contexts.
Animal Models
Animal experiments provide crucial insights into biological processes, particularly in aging and muscle injury studies. The protocols for such research are strictly regulated and follow guidelines set forth by the Institutional Animal Care and Use Committee (IACUC). For instance, in a study conducted at New York University’s Grossman School of Medicine, C57BL/6 J mice were selected, ranging in ages from young adults (3–6 months) to geriatric (28–31 months). These mice were male, thereby ensuring a sufficient statistical power in aged cohorts. They were housed under controlled light/dark conditions at a comfortable temperature of 21.2°C with 58% humidity. This baseline helps eliminate environmental variables that could skew research outcomes.
Cell Culture and Staining Techniques
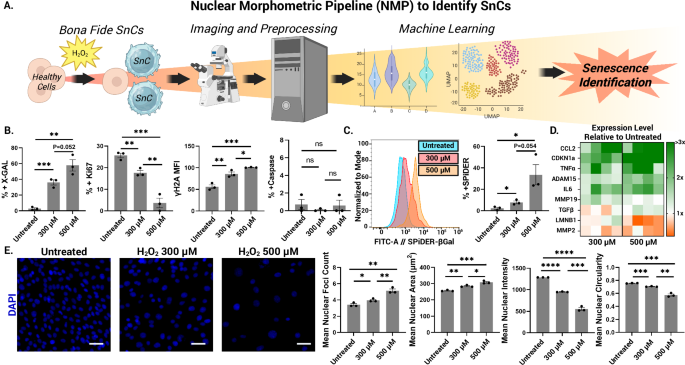
Cell culture serves as a cornerstone of laboratory procedures, facilitating the investigation of cellular behavior under various conditions. In particular, C2C12 and 3T3-L1 cells are commonly used in myogenic and adipogenic research, respectively. C2C12 cells, sourced from the esteemed researcher Stefano Biressi, are vital for exploring muscle cell behavior. They were cultured in a medium supplemented with fetal bovine serum and antibiotics, ensuring optimal growth and viability.
To induce cellular senescence, C2C12 cells were subjected to hydrogen peroxide (H₂O₂), etoposide, or doxorubicin treatments, creating a model to study the aging process at the cellular level. Staining methods, such as the Senescence β-Galactosidase Staining Kit, were then employed to quantify senescent cells accurately. Brightfield and fluorescent imaging techniques were utilized for both quantitative and qualitative analyses, capturing the essential data in a user-friendly manner through advanced microscopy.
For immunostaining protocols, fixed cells underwent permeabilization and were subsequently incubated with specific primary antibodies that targeted key cellular markers. This meticulous approach allows researchers to elucidate the presence and intensity of proteins associated with cellular function and aging.
RT-qPCR for Gene Expression Analysis
The extraction of total RNA from cultured cells is another fundamental procedure, allowing researchers to assess gene expression levels critically. Following protocols with products like the RNeasy Kit, scientists can utilize reverse transcription to generate cDNA, which serves as the template for real-time quantitative PCR (RT-qPCR). This technology facilitates a deeper understanding of how specific genes are expressed in various states, such as in response to treatments that induce senescence.
Muscle Injury Models
Muscle injuries provide a practical context for studying regeneration and repair mechanisms. In such experiments, mice are anesthetized with isoflurane, and a chemical agent like BaCl₂ is injected into the muscle. This deliberate injury enables researchers to investigate the underlying biological responses, with key focus areas being cell recruitment and muscle regeneration over time.
Cell Isolation and Fluorescence Activated Cell Sorting (FACS)
Following muscle injury, isolating specific cell types from muscle tissue is essential for analyzing their characteristics and responses to treatment. Techniques involving collagenase digestion allow for the extraction of single cells, which are then subjected to fluorescence-activated cell sorting (FACS). This method aligns the cells based on specific markers, facilitating the enrichment of populations such as endothelial cells, immune cells, and muscle satellite cells. The sorted cells can then be analyzed further to elucidate their roles in muscle recovery and fibrosis.
Skeletal Muscle Histology and Immunofluorescence
Histological techniques are invaluable for visualizing muscle integrity post-injury. Tibialis anterior muscles, for instance, are isolated and fixed for sectioning and staining, enabling assessment of myofiber damage or repair. Immunofluorescence approaches allow for the delineation of various cellular markers within these sections, contributing to an understanding of cellular organization and the impact of treatment interventions on muscle regeneration.
Analytics and Imaging Techniques
Advanced imaging systems, such as the Nikon Eclipse Ti2-LAPP, are pivotal in capturing high-resolution fluorescent images that reveal intricate cellular details. Image processing software enhances these images, preparing them for quantitative analysis. Techniques such as UMAP (Uniform Manifold Approximation and Projection) are implemented to visualize and cluster cellular phenotypic data, facilitating the exploration of biological variability and significance.
Conclusion
The integration of animal models, cell culture practices, and advanced analytical methods creates a multifaceted approach to investigating biological questions. By understanding the nuances of these techniques, researchers can draw meaningful conclusions that further our knowledge of health, disease mechanisms, and potential therapeutic interventions.