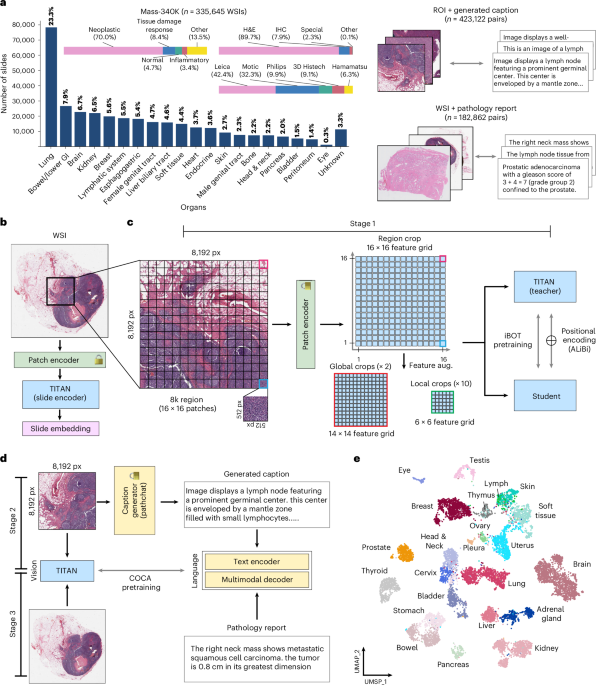
Revolutionizing Pathology: A Multimodal Whole-Slide Foundation Model
Understanding Multimodal Whole-Slide Imaging
Definition: Multimodal whole-slide imaging refers to the integration of different imaging techniques and data types—such as histopathological slides, molecular data, and clinical information—to enhance diagnostic accuracy and clinical decision-making.
Example: A pathologist uses a multimodal system that combines traditional hematoxylin and eosin (H&E) stained slides with advanced molecular imaging data to better identify cancerous tissues, leading to improved diagnostic confidence.
Structural Model: Considering the different modalities involved, this could be represented in a flow diagram that outlines how various data types (eg, imaging, genomic data, patient history) converge into a centralized diagnostic model.
Reflection: What assumption might a professional in pathology overlook here? Could the integration of molecular data distort or enhance the interpretation of traditional histological findings?
Application: Implement a platform that integrates various modalities, enhancing not just diagnostic accuracy but also enabling tailored treatment strategies based on comprehensive data analysis.
The Role of Foundation Models in Pathology
Definition: A foundation model is a pre-trained machine learning model that serves as a base for specific tasks within a domain, allowing for fine-tuning with relatively small datasets.
Example: In pathology, a foundation model trained on millions of pathology slides can be fine-tuned to detect specific subtypes of breast cancer, increasing efficiency and accuracy.
Structural Model: A side-by-side comparison table showcasing performance metrics of traditional machine learning models versus foundation models, highlighting improvements in diagnostic accuracy and reduction in training time.
Reflection: What would change first if this system began to fail in real conditions? Would it be the accuracy of the diagnostics or the user adoption rate among pathologists?
Application: Leverage foundation models to accelerate the development of specialized diagnostic tools that require less data while still achieving high-performance levels.
Challenges of Implementing Multimodal Models
Definition: Implementing multimodal models in pathology involves integrating diverse data types and ensuring compatibility, which can be technically and logistically challenging.
Example: A hospital may attempt to use genomic data alongside histological images but faces software compatibility issues, leading to delays in analysis.
Conceptual Diagram: A workflow diagram illustrating the steps involved in integrating multiple data types—from data acquisition and preprocessing to modeling and operational deployment.
Reflection: What assumptions exist about software interoperability in pathology that could lead to overlooked obstacles during integration?
Application: Establish guidelines for data integration that prioritize software compatibility, ensuring smoother transitions when implementing new multimodal AI systems.
Measuring Effectiveness: Metrics and Benchmarks
Definition: Effectiveness in multimodal pathology models can be measured using specific metrics such as diagnostic accuracy, false positive rates, and user satisfaction scores.
Example: A recent study might show that a new multimodal system improved diagnostic accuracy from 80% to 92% while reducing false positives from 10% to 4%.
Framework Comparison: A matrix comparing various metrics used in evaluating traditional vs. multimodal models, focusing on performance and operational efficiency.
Reflection: What common mistakes in metric selection might lead an organization to misjudge the success of their multimodal applications in pathology?
Application: Choose metrics strategically, ensuring they align with clinical goals—such as accuracy and speed—rather than solely focusing on technological capabilities.
Future Trends in Multimodal Pathology
Definition: Emerging trends in multimodal pathology include the increasing adoption of AI, real-time data analysis, and personalized medicine approaches.
Example: A hospital implements AI-powered real-time analysis that enables pathologists to receive instant diagnostic feedback from remote imaging analyses during surgeries.
Lifecycle Process: A lifecycle model illustrating the stages of multimodal pathology—from initial research and development through field trials to widespread clinical deployment.
Reflection: What long-term implications might arise for pathologists’ roles as automation and AI become more integrated into diagnostic processes?
Application: Stay ahead of the curve by investing in continuous education and training programs for pathologists, ensuring they are equipped to adapt to these technological advancements.
FAQ Section
Q: How long does it take to train a foundation model for pathology applications?
A: Training can vary, but leveraging pre-trained models can reduce the time significantly—down from months to weeks depending on the complexity of the task.
Q: Are there risks associated with the integration of AI in pathology?
A: Yes, potential risks include over-reliance on technology, data privacy concerns, and the need for robust validation to ensure that models are reliable in real-world conditions.
Q: What types of data are most applicable for multimodal models in pathology?
A: Common data types include imaging data (e.g., H&E slides), genomic profiles, and clinical metadata that provide comprehensive context for diagnoses.
Q: How can we evaluate the success of multimodal AI systems in a clinical setting?
A: Success can be gauged through specific metrics such as improved diagnostic accuracy, user satisfaction, and operational efficiency, helping to determine ROI on technology investments.