“Enhancing Preoperative Glioma Grading with Deep Learning and Radiomics from Multi-Center MRI Data”
Enhancing Preoperative Glioma Grading with Deep Learning and Radiomics from Multi-Center MRI Data
Key Concepts in Glioma Grading
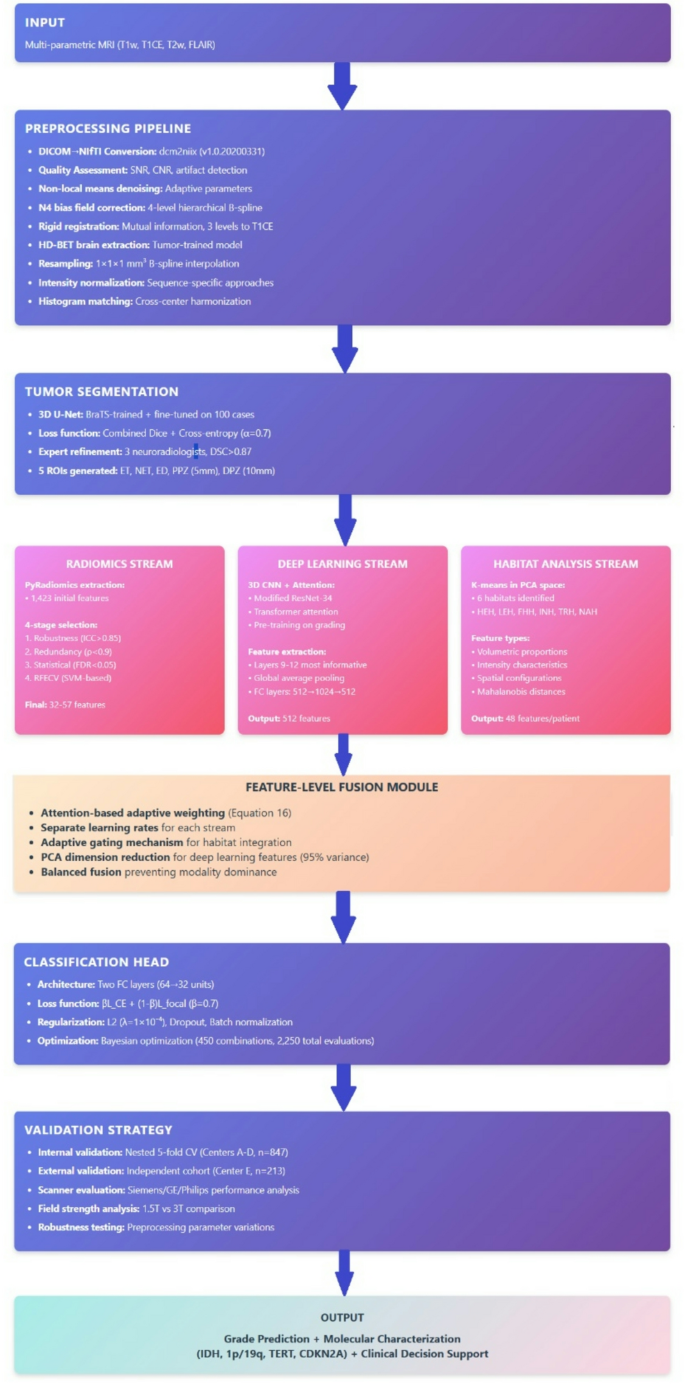
Gliomas are a type of brain tumor characterized by their origin from glial cells, and they vary widely in terms of biological behavior and patient prognosis. Accurate grading of gliomas—typically done preoperatively—can significantly affect treatment decisions and outcomes. Traditional grading relies on histopathological evaluation, but emerging technologies like deep learning and radiomics offer new avenues for analysis. Radiomics refers to the extraction of quantitative features from medical images, enabling a deeper understanding of tumor characteristics.
Importance of Preoperative Grading
Preoperative grading of gliomas is critical because treatment plans, including surgery and potential adjuvant therapies, often depend on the tumor’s grade. High-grade gliomas (like glioblastomas) have poorer prognoses than low-grade variants and require aggressive treatment. By accurately predicting glioma grades preoperatively, clinicians can better tailor their strategies, potentially improving patient outcomes and resource allocation.
The Role of Deep Learning
Deep learning is a subset of artificial intelligence that utilizes neural networks to analyze vast datasets and identify intricate patterns. In glioma grading, deep learning models can be trained using multiparametric MRI data to detect features that may not be visible to the human eye. For example, deep learning algorithms can discern subtle differences in tissue contrast and morphology, leading to more accurate grading compared to traditional methods.
Multi-Center MRI Data Utilization
Leveraging data from multiple centers allows for a more robust analysis due to the diversity in imaging protocols, patient demographics, and tumor characteristics. For instance, a study aggregated high-quality preoperative MRI scans from five neurosurgical centers, which provided a rich dataset for training and validating deep learning models. This approach reduces model overfitting, enhances generalizability, and ensures that findings are applicable across diverse clinical settings.
Steps in the Grading Process
-
Dataset Preparation: Involves standardizing the imaging protocols across multiple centers to ensure consistency. Centers utilize a range of MRI sequences, including T1-weighted, T1-weighted with contrast, T2-weighted, and FLAIR images.
-
Preprocessing: MRI data undergoes multiple preprocessing steps, including noise reduction and bias field correction, to mitigate variations across machines, enhancing the quality of subsequent analyses.
-
Feature Extraction: This step involves using algorithms to identify and quantify radiomic features from the MRI scans. These features include shape, texture, and intensity of the tumors, which are crucial for training predictive models.
-
Model Training: Deep learning models, such as convolutional neural networks (CNNs), are trained on the extracted features to classify tumor grades. High-performance computing resources enable faster and more efficient training processes.
- Validation: The models are rigorously validated using a separate dataset to ensure reliability and accuracy in grading decisions. External validation cohorts are crucial for testing the model’s robustness.
Example of Radiomic Features in Tumor Analysis
Radiomic features can include metrics such as shape (volume, surface area), texture (first-order statistics, co-occurrence matrices), and wavelet transform features that capture variations within the tumor. For instance, studies have shown that specific texture patterns may correlate with tumor aggressiveness. By integrating these features with deep learning algorithms, models can predict glioma grades with impressive accuracy, facilitating enhanced patient management strategies.
Common Pitfalls and Solutions
One common pitfall in using these technologies is the potential for overfitting, where models perform well on training data but poorly on unseen data. This can occur due to limited variability in training sets. To counter this, employing techniques such as cross-validation, data augmentation, and robust feature selection helps ensure the model generalizes well.
Additional Challenges
Imbalanced datasets pose another challenge, particularly if certain tumor grades are disproportionately represented. Techniques like Synthetic Minority Oversampling Technique (SMOTE) can help balance the dataset, ensuring that models can learn from all classes effectively.
Tools and Frameworks in Practice
Machine learning frameworks like TensorFlow and PyTorch are widely used for developing deep learning models. With these tools, researchers can construct complex neural networks tailored to specific tasks such as glioma grading. Radiomics software, such as PyRadiomics, standardizes feature extraction, which ensures reproducibility across studies.
Alternative Approaches and Their Trade-offs
While deep learning offers powerful capabilities, traditional statistical methods and hand-crafted radiomic features still have merits. These approaches often provide interpretability, allowing clinicians to understand the reasoning behind grading decisions. However, deep learning models may capture intricate relationships that traditional models cannot.
Hybrid methodologies, which combine both approaches, leverage the strengths of each—offering explainability with data-driven insights.
FAQs
-
What are the benefits of using deep learning in glioma grading?
- Deep learning automates the feature extraction process and can identify complex patterns that may be missed in traditional analysis, leading to more accurate grading.
-
How does multi-center data improve model performance?
- Multi-center data ensures greater variability, making models more robust and applicable across different clinical settings.
-
What preprocessing techniques are critical for MRI data?
- Denoising, bias field correction, and intensity normalization are essential for minimizing artifacts and enhancing image quality before analysis.
- How can feature selection influence model accuracy?
- Effective feature selection reduces noise, avoids overfitting by focusing on the most relevant features, and enhances the model’s predictive power.
This comprehensive approach to integrating deep learning and radiomics represents a significant leap forward in the preoperative grading of gliomas, providing tools that can dramatically impact clinical outcomes.